DOI:10.19476/j.ysxb.1004.0609.2017.08.22
氯化钠在蛇纹石/黄铁矿浮选体系中的分散机理
刘 诚,冯其明,石 晴,张国范,陈延飞
(中南大学 资源加工与生物工程学院,长沙 410083)
摘 要:
通过矿物浮选实验、沉降实验、动电位和DLVO理论计算,考察氯化钠在蛇纹石/黄铁矿浮选体系中的聚集/分散作用,研究氯化钠对颗粒间的分散作用机制。结果表明:蛇纹石颗粒可通过异相凝聚作用罩盖在黄铁矿表面,阻碍黄药在黄铁矿表面吸附,影响黄铁矿的浮选;氯化钠的加入能增加蛇纹石与黄铁矿颗粒间的分散性,提高黄铁矿/蛇纹石混合矿体系中黄铁矿的回收率。研究结果表明,在pH为8.5时,氯化钠的浓度越高,对混合矿颗粒间的分散性越强;蛇纹石通过静电作用吸附在黄铁矿表面影响其浮选,氯化钠加入压缩双电层,使带相反电性的颗粒间电位绝对值显著降低,降低静电引力,使异相凝聚程度减弱,降低蛇纹石对黄铁矿的抑制作用。
关键词:
文章编号:1004-0609(2017)-08-1715-05 中图分类号:TD91 文献标志码:A
金属镍被称为“工业维生素”,是生产各种类型钢和合金非常重要的原材料[1],而硫化铜镍矿是目前世界上镍的最主要来源,一般硫化铜镍矿中,主要的有用矿物为黄铜矿、镍黄铁矿、磁黄铁矿和黄铁矿等,矿石中脉石主要是含镁硅酸盐,包括蛇纹石,滑石和绿泥石等[2-6]。诸多矿物加工研究者通过动电位、扫描电镜、原子力显微镜、沉降等研究手段表明,蛇纹石在浮选的过程中易泥化,并通过静电吸附作用于硫化镍矿物表面,降低硫化矿物的浮选回收率,因此,消除蛇纹石在硫化矿物表面的罩盖,降低蛇纹石对硫化矿物浮选的影响,对高效利用铜镍资源具有重要意义[6-8]。
目前,常用的蛇纹石与硫化镍矿物分散剂主要有六偏磷酸钠、羧甲基纤维素(CMC)等[7-9],这些分散剂能显著降低蛇纹石矿物表面的电位并消除异相凝聚来提高铜镍回收率,但大部分研究都基于淡水(去离子水或蒸馏水)体系中硫化矿物与蛇纹石之间的异相凝聚而进行的表面电性调控[10-14]。而关于盐水体系中蛇纹石对硫化镍矿浮选的影响的报道较少,PENG等[15]和CAO等[16]研究表明:盐水浮选体系中可以显著改善蛇纹石对硫化镍矿浮选过程产生的不利影响,然而,盐水体系中对硫化镍矿与蛇纹石颗粒之间的聚集/分散及相互作用机理认识并不深入。因此,本文作者通过矿物浮选实验、沉降实验、Zeta电位测试和DLVO理论计算,考察盐水体系中蛇纹石/黄铁矿的聚集/分散作用,研究盐水体系黄铁矿与蛇纹石颗粒间作用机理,为进一步研究盐水体系中消除蛇纹石对黄铁矿的影响提供技术参考与理论依据。
1 实验
1.1 矿物样品与试剂
金川硫化铜镍矿的主要硫化矿物有镍黄铁矿、黄铁矿、磁黄铁矿,但纯净的镍黄铁矿难以制取,因此本文选用表面性质相近的黄铁矿作为硫化矿物的代表。黄铁矿取自广东云浮,蛇纹石取自江苏东海。实验用纯矿物制备方法为:人工选取结晶度较好块矿,经锤碎手选后用瓷球磨、搅拌磨磨细。经筛分得到 37~74 μm 粒级的黄铁矿及水析得到粒径小于10 μm的蛇纹石颗粒用于实验试样。实验用氯化钠(NaCl)浓度来表示溶液体系的盐度,以丁基黄药(SBX)为捕收剂、甲基异丁基甲醇(MIBC)为起泡剂、盐酸(HCl)、氢氧化钠(NaOH)为pH调整剂,以上药剂均为分析纯,实验用水为一次蒸馏水。
1.2 研究方法
1.2.1 纯矿物浮选实验
浮选实验采用40 mL的XFG型挂槽式浮选机。每次实验称取表面清洁的黄铁矿样品2 g,并加0.1 g蛇纹石至浮选槽中,加40 mL蒸馏水搅拌1 min,加氯化钠(可变)搅拌2 min,加调整剂搅拌2 min,加捕收剂搅拌3 min,加MIBC搅拌1 min。测定pH值后,浮选过程采取手工刮泡5 min。将所得的泡沫产品与槽内产品烘干、称量,经化学分析后计算黄铁矿的浮选回收率。
1.2.2 沉降实验
采用矿浆的浊度表征颗粒间的分散性,浊度越大,其分散性越好。沉降实验在100 mL 比色管中进行,黄铁矿质量浓度10 g/L,蛇纹石质量浓度为1 g/L按浮选实验条件调浆后,倒入比色管沉降3 min,抽取上部25 mL 悬浮液,用WGZ-3型散射光浊度仪测定悬浮液浊度。
1.2.3 Zeta电位测试
采用Coulter Delsa440sx型Zeta电位分析仪进行Zeta电位测试。将蛇纹石和黄铁矿纯矿物细磨至粒径小于2 μm,用高精度天平称取30 mg,放入烧杯中并加入50 mL蒸馏水,调节溶液pH值并添加浮选药剂后搅拌3 min,然后放入样品池中进行Zeta电位测定,每个实验条件测量3次后取平均值。实验所用电解质为1×10-3 mol/L的KNO3溶液。
2 结果与讨论
2.1 氯化钠对黄铁矿与蛇纹石浮选分离的影响
本实验中黄铁矿粒度较粗,在实验条件和所测定的pH值范围内,黄铁矿易沉降,浊度极低,因此,可以用蛇纹石单矿物的浊度值表征混合矿的理论浊度。图1所示为氯化钠对蛇纹石与黄铁矿人工混合矿分散凝聚的影响。由图1可知,蛇纹石与黄铁矿混合矿的浊度比蛇纹石单矿物的浊度小,说明黄铁矿与蛇纹石之间发生了异相凝聚[17],随矿浆 pH 升高,蛇纹石与黄铁矿异相凝聚行为加剧。加入氯化钠后,基本不影响蛇纹石单矿物的浊度,而在pH为7~11区间 时,人工混合矿浊度升高,这说明在pH为7~11的区间内,氯化钠的添加对黄铁矿与蛇纹石混合矿具有分散作用。
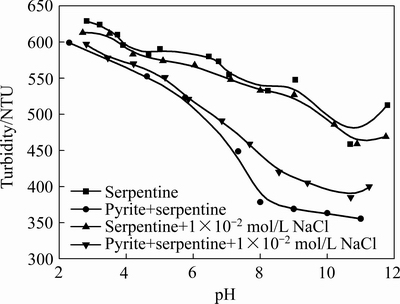
图1 氯化钠对单矿物及混合矿浊度的影响
Fig. 1 Effect of sodium chloride on turbidity of single ore and mixed ore
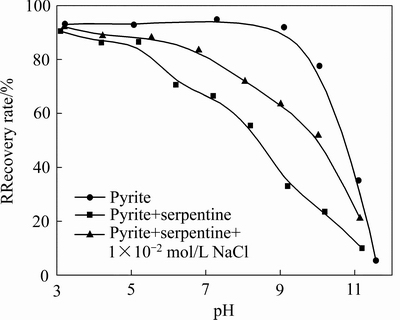
图2 黄铁矿浮选回收率随pH值变化(SBX浓度1×10-4 mol/L,MIBC浓度1×10-4 mol/L)
Fig. 2 Flotation recovery of pyrite as a function of pH at different condition (SBX concentration of 1×10-4 mol/L, MIBC concentration of 1×10-4 mol/L)
图2所示为不同条件下黄铁矿浮选回收率随pH值的变化曲线。由图2可知,单一黄铁矿可浮性在pH小于10时都表现出良好的可浮性;当加入0.1 g蛇纹石时,黄铁矿的回收率随pH值升高呈明显下降趋势,结合图1说明异相凝聚影响黄药在黄铁矿表面吸附;当在黄铁矿/蛇纹石体系中加入1×10-2 mol/L氯化钠时,在pH为6~10区间黄铁矿回收率显著提高。
为考查氯化钠对黄铁矿/蛇纹石体系聚集分散及浮选分离行为影响,进行氯化钠用量实验,图3所示为pH 8.5时蛇纹石及黄铁矿与蛇纹石混合矿浊度随氯化钠用量变化。由图3可知,蛇纹石浊度随氯化钠浓度增加呈小幅减少趋势,基本不影响蛇纹石的聚集/分散状态;黄铁矿与蛇纹石混合矿浊度随氯化钠浓度增加而增加,说明氯化钠浓度增加有利于黄铁矿与蛇纹石颗粒间分散,结合图4中氯化钠浓度对黄铁矿回收率的影响可知,黄铁矿的回收率变化趋势与混合矿体系的浊度相对应,表明添加氯化钠能减少蛇纹石在黄铁矿表面罩盖,提高黄铁矿浮选回收率。
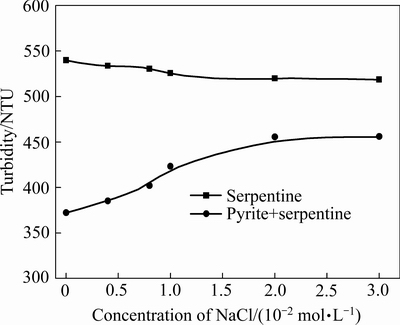
图3 氯化钠浓度对蛇纹石及混合矿浊度的影响(pH=8.5)
Fig. 3 Effect of sodium chloride concentration on turbidity of serpentine and mixed ore at pH 8.5
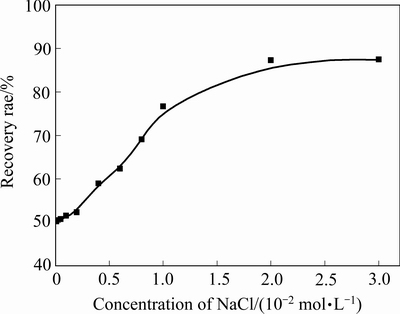
图4 氯化钠浓度对蛇纹石存时黄铁矿回收率的影响(SBX浓度1×10-4 mol/L,MIBC浓度1×10-4 mol/L,pH=8.5)
Fig. 4 Effect of sodium chloride concentration on flotation of pyrite in presence of serpentine (SBX concentration of 1×10-4 mol/L, MIBC concentration of 1×10-4 mol/L, pH=8.5)
2.2 矿物表面电性及相互作用分析
当pH为8.5时,蛇纹石与黄铁矿表面Zeta电位随氯化钠浓度的变化如图5所示。由图 5可知:氯化钠浓度为0 mol/L,蛇纹石表现出强烈的荷正电,黄铁矿表现出强烈的荷负电,两者电性相反,通过静电吸引作用发生异相凝聚,使蛇纹石罩盖在黄铁矿表面,影响黄铁矿的浮选,当氯化钠浓度增加至2×10-2 mol/L,黄铁矿和蛇纹石的动电位绝对值逐渐减小且趋近于零,继续增大氯化钠浓度,黄铁矿和蛇纹石表面Zeta电位均没有出现明显变化,表明氯化钠不是以吸附的形式作用于矿物表面。由双电层理论可知,添加电解质氯化钠时,新增的反离子与扩散层内原有反离子之间的静电斥力将原有反离子压缩进紧密层,使扩散层变薄,颗粒表面Zeta电位绝对值降低,黄铁矿与蛇纹石颗粒间的静电引力减小[18]。
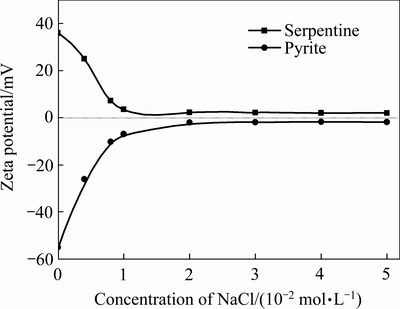
图5 氯化钠浓度对矿物表面电性的影响(pH=8.5)
Fig. 5 Effect of sodium chloride concentration on Zeta potential of minerals surface at pH 8.5
根据经典DLVO理论[19-21],颗粒的聚集/分散主要由矿物颗粒之间范德华力和静电作用力决定。矿物表面动电位绝对值变化必然会影响矿物颗粒之间的静电作用能,从而影响颗粒之间的聚集/分散状态。异相矿物颗粒间相互作用总势能 VT可表征为
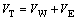 (1)
(1)
式中:VW为范德华作用能;VE为静电作用能。
 (2)
(2)
式中:H为两颗粒间距离,单位为nm;A为Hamaker常数;R为颗粒半径,这里分别取R1为5 μm,R2为40 μm。颗粒1和颗粒2在介质3中相互作用的Hamaker常数由式(3)给出:
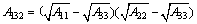 (3)
(3)
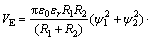
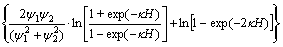 (4)
(4)
式中:A11为颗粒在真空中的Hamaker常数;A33为介质在真空中的Hamaker常数;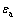 是水的介电常数,为6.95×10-10 C2/(J·m);T为热力学温度,单位为K;
是水的介电常数,为6.95×10-10 C2/(J·m);T为热力学温度,单位为K;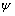 为矿物的表面电位,单位为V;H为两颗粒间距离,单位为nm;
为矿物的表面电位,单位为V;H为两颗粒间距离,单位为nm;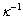 为Debye长度,单位为nm,代表双电层厚度。
为Debye长度,单位为nm,代表双电层厚度。
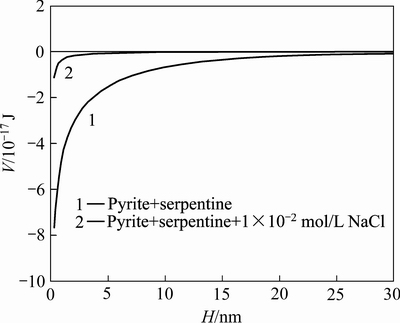
图6 黄铁矿与蛇纹石颗粒间相互作用总势能与颗粒间距H之间的关系
Fig. 6 Relationship between interaction energy of pyrite-serpentine particles and particles distance
根据式(1)可以得知,pH=8.5时矿物颗粒间相互作用总势能与颗粒间距的关系,如图6所示。由图6可知,黄铁矿与蛇纹石颗粒间的相互作用能为负值,存在强烈吸引作用,容易发生异相凝聚。当加入氯化钠后,黄铁矿与蛇纹石之间引力势能急剧降低,表明颗粒间的静电引力急剧减小,颗粒间主要表现为范德华力,而黄铁矿/蛇纹石浮选过程中,矿粒在流体力场中运动并具有一定的动能,容易克服颗粒间的范德华力使颗粒间表现出分散状态,提高黄铁矿的浮选回收率。
3 结论
1) 含镍硫化矿物常在弱碱性下浮选,在此pH区间,蛇纹石易罩盖在黄铁矿表面,影响了黄铁矿的浮选;氯化钠的添加能减弱蛇纹石与黄铁矿之间的异相凝聚,改善蛇纹石对黄铁矿产生的不利影响。
2) 氯化钠通过压缩双电层来改变蛇纹石与黄铁矿表面的电性,氯化钠浓度越高,蛇纹石与黄铁矿表面电位绝对值越小。
3) 当加入氯化钠时,蛇纹石与黄铁矿颗粒之间的相互作用能由强烈的吸引变为微弱的吸引,颗粒间异相凝聚程度降低。
REFERENCES
[1] LU Ji-wei, YUAN Zhi-tao, LIU Jiong-tian, LI Li-xi, ZHU Shuo. Effects of magnetite on magnetic coating behavior in pentlandite and serpentine system[J]. Minerals Engineering, 2015, 72: 115-120.
[2] 卢毅屏, 龙 涛, 冯其明, 欧乐明, 张国范. 微细粒蛇纹石的可浮性及其机理[J]. 中国有色金属学报, 2009, 19(8): 1493-1497.
LU Yi-ping, LONG Tao, FENG Qi-ming, OU Le-ming, ZHANG Guofan. Flotation and its mechanism of fine serpentine[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(8): 1493-1497.
[3] Malysiak V, O'Connor C T, Ralston J, Gerson A R, Coetzer L P, Bradshaw D J. Pentlandite–feldspar interaction and its effect on separation by flotation[J]. International Journal of Mineral Processing, 2002, 66(1/4): 89-106.
[4] FORNASIO D, RALSTON J. Cu(Ⅱ) and Ni(Ⅱ) activation in the flotation of quartz, serpentine and chlorite[J]. International Journal of Mineral Processing, 2005, 76(1/2): 75-81.
[5] CHEN G, GRANO S, SOBIERAJ S, et al. The effect of high intensity conditioning on the flotation of a nickel, part 2: Mechanisms[J]. Minerals Engineering, 1999, 12(11): 1359-1373.
[6] 卢毅屏, 张明洋, 冯其明, 欧乐明, 张国范. 蛇纹石与滑石的同步抑制原理[J]. 中国有色金属学报,2012,22(2): 560-565.
LU Yi-ping, ZHANG Ming-yang, FENG Qi-ming, OU Le-ming, ZHANG Guo-fan. Sync-suppression principle of serpentine and talc[J]. The Chinese Journal of Nonferrous Metals, 2012,22(2): 560-565.
[7] 卢毅屏, 丁 鹏, 冯其明, 欧乐明,张国范. 不同结构的磷酸盐对蛇纹石的分散作用[J]. 中南大学学报(自然科学版), 2011, 42(12): 3599-3604.
LU Yi-ping, DING Peng, FENG Qi-ming, OU Le-ming, ZHANG Guo-fan. Dispersion effect of different structural phosphates on serpentine[J]. Journal of Central South University (Science and Technology), 2011, 42(12): 3599-3604.
[8] 冯 博, 冯其明, 卢毅屏. 羧甲基纤维素在蛇纹石/黄铁矿浮选体系中的分散机理[J]. 中南大学学报(自然科学版), 2013, 44(7): 1933-1939.
FENG Bo, FENG Qi-ming, LU Yi-ping. Dispersion mechanism of CMC on flotation system of serpentine and pyrite[J]. Journal of Central South University (Science and Technology), 2013, 44(7): 1933-1939.
[9] BremmelL K E, Fornasiero D, Ralston J. Pentlandite–lizardite interactions and implications for their separation by flotation[J]. Colloids and Surfaces A, 2005, 252: 207-212.
[10] Wellham E J, Elber L, Yan D S. The role of carboxy methyl cellulose in the flotation of a nickel sulphide transition ore[J]. Minerals Engineering, 1992, 5(3/5): 381-395.
[11] Pietrobon M C, Grano S R, Sobieraj S,Ralston J. Recovery mechanisms for pentlandite and MgO-bearing gangue minerals in nickel ores from western Australia[J]. Minerals Engineering, 1997, 10(8): 775-786.
[12] Basile A, Hughes J, McFarlane A J, Bhargava S K. Development of a model for serpentine quantification in nickel laterite minerals by infrared spectroscopy[J]. Minerals Engineering, 2010, 23(5): 407-412.
[13] Kirjavainen V, Heiskanen K. Some factors that affect beneficiation of sulphide nickel-copper ores[J]. Minerals Engineering, 2007, 20(7): 629-633.
[14] Senior G D, Thomas S A. Development and implementation of a new flowsheet for the flotation of a low grade nickel ore[J]. Int J Miner Process, 2005, 78(1): 49-61.
[15] PENG Y J, BRADSHAW D. Mechanisms for the improved flotation of ultrafine pentlandite and its separation from lizardite in saline water[J]. Minerals Engineering, 2012, 36/38: 284-290.
[16] CAO Jian, HU Xiu-qin, LUO Yong-chun, QI Li, XU Guo-qiang, XU Peng-fei. The role of some special ions in the flotation separation of pentlandite from lizardite[J]. Colloids and Surfaces A, 2016, 490: 173-181.
[17] 龙 涛, 冯其明, 卢毅屏. 六偏磷酸钠在硫化铜镍矿浮选中的分散机理[J]. 中国有色金属学报, 2012, 22(6): 1763-1769.
LONG Tao, FENG Qi-ming, LU Yi-ping. Dispersive mechanism of sodium hexametaphosphate on flotation of copper nickel sulphide[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(6): 1763-1769.
[18] BREMMELL K E, FORNASIERO D, RALSTON J. Pentlandite-lizardite interactions and implications for their separation by flotation[J]. Colloids and Surfaces A, 2005, 252: 207-212.
[19] LU Yi-ping, ZHANG Ming-qiang, FENG Qi-ming, LONG Tao, OU Le-ming, ZHANG Guo-fan. Effect of sodium hexametaphosphate on separation of serpentine from pyrite[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(1): 208-213.
[20] ADAMCZYK P,  P. Application of the DLVO theory for particle deposition problems[J]. Advances in Colloid and Interface Science, 1999, 83(1/3): 137-226.
P. Application of the DLVO theory for particle deposition problems[J]. Advances in Colloid and Interface Science, 1999, 83(1/3): 137-226.
[21] Missnan T, Adell A. On the applicability of DLVO theory to the prediction of clay colloids stability[J]. Journal of Colloid and Interface Science, 2000, 230(1): 150-156.
Dispersion mechanism on flotation system of serpentine and pyrite in presence of sodium chloride
LIU Cheng, FENG Qi-ming, SHI Qing, ZHANG Guo-fan, CHEN Yan-fei
(School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The aggregation/dispersion effect of sodium chloride on flotation system of serpentine and pyrite and its mechanism were studied by flotation experiments, sedimentation tests, zeta potential and calculations of DLVO theory. The results show that the serpentine particles shroud on pyrite surface by hetero-aggregation, depress the adsorption of xanthate on pyrite surface and decrease the flotation recovery rate of pyrite. The dispersion between particles of the serpentine and pyrite increases in the presence of sodium chloride. The higher concentration of sodium chloride is added, the higher dispersion exists between the particles. Sodium chloride adjusts the surface potential of serpentine through compressing double electrode layer and then disperses serpentine and pyrite, then improving the flotation performance of pyrite.
Key words: pyrite; serpentine; sodium chloride; dispersion; mechanism
Foundation item: Project(2014CB643402) supported by the National Basic Research Development Program of China; Project(2016zzts472) supported by the Innovation Project of Central South University, China; Project (301170302) supported by the State Key Laboratory of Nickel and Cobalt Resources Comprehensive Utilization, China
Received date: 2016-07-15; Accepted date: 2016-12-30
Corresponding author: SHI Qing; Tel: +86-731-8830913; E-mail: 330595812@qq.com
(编辑 龙怀中)
基金项目:国家重大基础研究发展计划资助项目(2014CB643402);中南大学研究生创新资助项目(2016zzts472);镍钴资源综合利用国家重点实验室开放基金资助项目(301170302)
收稿日期:2016-07-15;修订日期:2016-12-30
通信作者:石 晴,讲师,博士;电话:0731-88830913;E-mail: 330595812@qq.com
摘 要:通过矿物浮选实验、沉降实验、动电位和DLVO理论计算,考察氯化钠在蛇纹石/黄铁矿浮选体系中的聚集/分散作用,研究氯化钠对颗粒间的分散作用机制。结果表明:蛇纹石颗粒可通过异相凝聚作用罩盖在黄铁矿表面,阻碍黄药在黄铁矿表面吸附,影响黄铁矿的浮选;氯化钠的加入能增加蛇纹石与黄铁矿颗粒间的分散性,提高黄铁矿/蛇纹石混合矿体系中黄铁矿的回收率。研究结果表明,在pH为8.5时,氯化钠的浓度越高,对混合矿颗粒间的分散性越强;蛇纹石通过静电作用吸附在黄铁矿表面影响其浮选,氯化钠加入压缩双电层,使带相反电性的颗粒间电位绝对值显著降低,降低静电引力,使异相凝聚程度减弱,降低蛇纹石对黄铁矿的抑制作用。
[2] 卢毅屏, 龙 涛, 冯其明, 欧乐明, 张国范. 微细粒蛇纹石的可浮性及其机理[J]. 中国有色金属学报, 2009, 19(8): 1493-1497.
[6] 卢毅屏, 张明洋, 冯其明, 欧乐明, 张国范. 蛇纹石与滑石的同步抑制原理[J]. 中国有色金属学报,2012,22(2): 560-565.
[7] 卢毅屏, 丁 鹏, 冯其明, 欧乐明,张国范. 不同结构的磷酸盐对蛇纹石的分散作用[J]. 中南大学学报(自然科学版), 2011, 42(12): 3599-3604.
[8] 冯 博, 冯其明, 卢毅屏. 羧甲基纤维素在蛇纹石/黄铁矿浮选体系中的分散机理[J]. 中南大学学报(自然科学版), 2013, 44(7): 1933-1939.
[17] 龙 涛, 冯其明, 卢毅屏. 六偏磷酸钠在硫化铜镍矿浮选中的分散机理[J]. 中国有色金属学报, 2012, 22(6): 1763-1769.


