Trans. Nonferrous Met. Soc. China 23(2013) 3306-3311
Preparation of TiO2/ITO film by liquid phase deposition and its photoelectrocatalytic activity for degradation of 4-aminoantipyrine
Dan LI, Hai-xia TONG, Ling ZHANG
Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, School of Chemistry and Biological Engineering, Changsha University of Science and Technology, Changsha 410004, China
Received 12 October 2012; accepted 11 April 2013
Abstract:
A thin layer of TiO2 film was deposited on ITO surface via the liquid phase deposition (LPD) process. The photocurrent and electrochemical impedance spectroscopy (EIS) measurements indicated that the as-prepared LPD TiO2/ITO film had an excellent photoelectrochemical performance, which showed a sensitive and rapid response to the UV irradiation. The photogenerated electron-hole pairs could be effectively separated by applying an external bias to the TiO2 film electrode. The LPD TiO2/ITO film was employed to study the photoelectrocatalytic (PEC) degradation of 4-aminoantipyrine. Compared with other techniques, the PEC technique based on such a LPD film electrode had a synergetic effect for 4-aminoantipyrine degradation. When the applied bias potential was +0.8 V and the supporting electrolyte concentration of Na2SO4 was 0.1 mol/L, the highest degradation efficiency within 120 min could reach 95% for 0.1 mmol/L 4-aminoantipyrine solution at pH 2.0.
Key words:
liquid phase deposition; TiO2 film electrode; photoelectrocatalysis; 4-aminoantipyrine;
1 Introduction
As one of the most important advanced oxidation techniques for degrading non-biodegradable pollutants, photoelectrocatalysis (PEC) has been the active research field in environmental science. In PEC, TiO2 film electrodes have been extensively utilized [1], due to the high photocatalytic activity and excellent stability of TiO2 catalyst. There have been many methods for the preparation of TiO2 films, such as sol-gel [2,3], microplasma oxidation [4], plasma surface alloying technique [5], magnetron sputtering [6,7], atomic layer deposition [8], metal-organic chemical vapor deposition [9] and electrochemical anodic oxidation [10]. DEKI et al [11] developed a new wet-chemical method, namely, liquid phase deposition (LPD), for the preparation of TiO2 film. In this method, substrate is immersed in aqueous solution of (NH4)2TiF6, followed by adding H3BO3 solution. After a period of reaction time, a layer of TiO2 film can be deposited on the substrate surface according to the following reactions.
 +nH2O
+nH2O
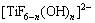 +nHF (1)
+nHF (1)
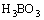 +4HF
+4HF HBF4+3H2O (2)
HBF4+3H2O (2)
Compared with the traditional methods for the preparation of TiO2 film, such a LPD technique has many advantages, such as simplicity, flexibility, low cost and no requirement of special device or substrate. Thus, LPD technique has been widely studied and utilized [12]. In the PEC degradation of pollutant, HOU et al [13] prepared TiO2 film on active carbon fiber by the LPD process, which has been successfully applied to the removal of acid orange II. DING et al [14] prepared LPD TiO2 film on glassy carbon electrode surface for the PEC degradation of benzotriazole.
Pharmaceuticals and personal care products (PPCPs) have been recently detected in environmental system and recognized as emerging contaminants. Because many PPCPs have the properties of good water solubility, high biological activity, high polarity, optical rotation and non-volatility, which threaten the safety of environmental eco-system and the health of human beings, the environmental problems of PPCPs have received considerable attention [15]. How to dispose such emerging contaminants has been an important content of environmental research [16]. 4-aminoantipyrine is a derivative of antipyrine. It is also the metabolic intermediate of dipyrone, one of the most widely used analgesic and antipyretic medicine. Because 4-aminoantipyrine is not completely removed by the traditional biochemical process in the wastewater treatment plants, it appeared in water environment as one of the most frequently detected PPCPs [17]. The recent report has demonstrated that the technique of UV/H2O2 could provide high degradation efficiency for 4-aminoantipyrine [18]. However, in the UV/H2O2 process, large amount of H2O2 is consumed. It is necessary to add H2O2 every some period of treatment time to maintain the high degradation efficiency. In the present work, we utilized optically transparent ITO electrode as substrate to deposit TiO2 film by the LPD process. The photoelectrochemical measurements indicated that the obtained TiO2/ITO film electrode had high PEC activity. Considering that TiO2/ITO film electrodes were useful to eliminate organic contaminants in wastewater [19,20], the as-prepared LPD TiO2/ITO film electrode was employed to systematically study the PEC degradation of 4-aminoantipyrine. The results showed that such a LPD TiO2/ITO film electrode could provide high PEC degradation efficiency for 4-aminoantipyrine under optimized conditions.
2 Experimental
2.1 Preparation of TiO2/ITO film
The TiO2 films were prepared on ITO (In2O3:Sn) conducting glass (10-15 Ω/?, 2 mm thick, 2 cm×2 cm) by the LPD process. Before deposition, ITO was cleaned by sonication in ethanol and water for 10 min. After being dried with a stream of high purity nitrogen gas, the ITO substrate was soaked vertically into the mixed aqueous solution of 0.1 mol/L (NH4)2TiF6 and 0.3 mol/L H 3BO3 at 50 °C for 10 h. After being cleaned with water and dried with nitrogen, the film was calcinated under 350 °C for 1 h.
2.2 PEC degradation of 4-aminoantipyrine
The PEC degradation of 4-aminoantipyrine was performed in a cylindrical reactor containing 150 mL 4-aminoantipyrine solution. A 15W low pressure UV lamp with a major emission wavelength of 254 nm was used as the illumination resource, which was protected in a quartz tube and then inserted into the center of the degradation solution. The PEC degradation experiments were controlled with a CHI660A electrochemical workstation (Shanghai Chenhua Instrument Co., China) using conventional three-electrode system. A TiO2/ITO film electrode, a platinum plate and a saturated calomel electrode (SCE) were inserted into the reactor to be employed as the working, counter and reference electrodes, respectively. The distance between the UV lamp and TiO2/ITO film was about 2 cm. During the degradation experiments, air was purged into the solution using an air pump with the flow rate of 3.0 L/min.
2.3 Characterization and analytical methods
The surface morphology of the TiO2 film was characterized with a field emission scanning electron microscopic (SEM) instrument (JSM-7400F, JEOL, Japan). The X-ray diffraction (XRD) analysis was carried out in an X’Pert PRO diffractometer (PANalytical B.V., Netherlands) using Cu Ka radiation. The concentration of 4-aminoantipyrine was analyzed at the maximum absorption wavelength of 4-aminoantipyrine at 243 nm (Fig. 1) with a UV-visible spectrometer (WFZ UV-2000, Unico Instruments Co., Ltd., Shanghai, China).
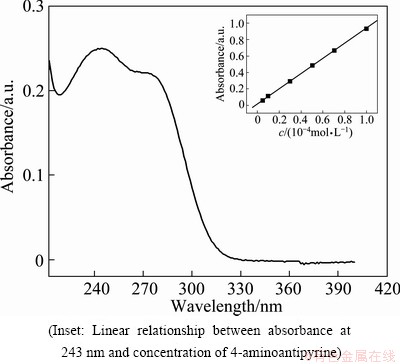
Fig. 1 UV-visible spectrum of 1.0×10-4 mol/L 4-amino- antipyrine
3 Results and discussion
3.1 Surface characterization of LPD TiO2 film
To charcterize the TiO2 film deposited on the ITO surface by the LPD process, the obtained film was observed by SEM. From Fig. 2, it is clearly observed that a layer of compact film is formed on the surface of ITO, which is similar to the LPD film prepared on the glassy carbon surface [14]. However, there appear many cracks in the film due to the internal stress of film during the drying process. The thickness of the deposited TiO2 film is ca. 400 nm, measured at cross-section of the film by SEM (Fig. 2(b)). The XRD analysis (Fig. 3) for calcinated film confirmes that the LPD film is composed of TiO2 (anatase) particles [14].
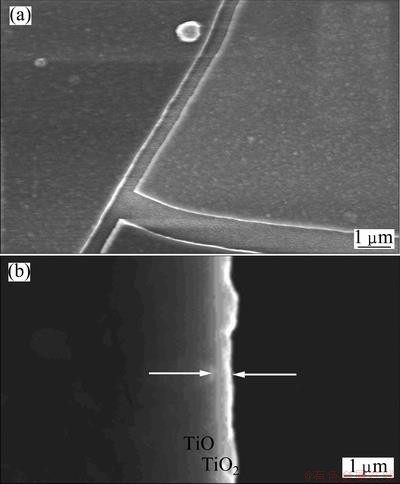
Fig. 2 Surface (a) and cross-section (b) SEM images of LPD TiO2/ITO film
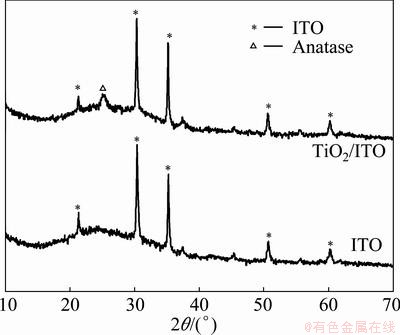
Fig. 3 XRD patterns of TiO2/ITO film and ITO substrate
3.2 Photoelectrochemical property of TiO2/ITO film
Figure 4 shows the photocurrent response of TiO2/ITO film electrode at 0.8 V under UV irradiation. As can be seen, the LPD TiO2 film electrode shows a fast and sensitive photochemical response to the UV light irradiation. The dark current without UV irradiation is only 8×10-8 A; whereas under UV irradiation, the photocurrent is quickly improved to 1.3×10-4 A. The large photocurrent is attributed to the photogenerated electrons on the TiO2 film driven to the counter electrode by the applied bias potential, demonstrating the excellent photoelectrochemical property of LPD TiO2/ITO film.
At the same time, in order to further investigate the photoelectrochemical property of such a LPD TiO2/ITO film electrode, electrochemical impedance spectoscopic (EIS) measurements were carried out before and after UV irradiation, as shown in Fig. 5. From Fig. 5, the electron transfer resistance (Rt) was calulated by fitting the diamter of semicircle appearing at the high frequency part of EIS using Zview software. When only bias potential is applied, the Rt value is 1685 kW. The large Rt value is assigned to the low conductivity of the compact semicondcutive film. While UV irrdiation and bias potential are simultaneously applied, the Rt value is remarkably decreased to 34 kW, meaning the promoted electron transfer in the electrode interface. The fast electron transfer is attributed to the effective separation of photogenerated electron-hole pairs on TiO2 by applied bias potential.
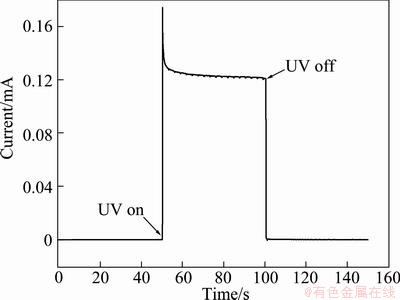
Fig. 4 Photocurrent response curve of LPD TiO2/ITO film electrode under UV irradiation

Fig. 5 Electrochemical impedance spectra (square) and corresponding fitting curves (line) of LPD TiO2/ITO film electrode with and without UV irradiation
3.3 PEC degdradation of 4-aminoantipyrine on TiO2/ITO film
The LPD TiO2/ITO film electrode was employed to study the PEC degradation of 4-aminoantipyrine. Figure 6 compares the degradation curves of 4-aminoantipyrine treated by different techniques such as electrolysis (EC), direct photolysis (DP), photocatalysis (PC) and PEC. In all these degradation experiments, the initial concentration of 4-aminoantipyrine was 0.1 mmol/L and the supporting electrolyte was 0.1 mol/L Na2SO4. The EC process was carried out by applying +0.8 V anodic bias potential on the TiO2/ITO film electrode without UV irradiation. The DP process was carried out under UV irradiation in the absence of film electrode and bias potential. The PC process was carried out under UV irradiation on the film electrode, but without applying bias potential. The PEC process was carried out under UV irradiation on the film electrode and simulataneously applying +0.8 V bias potential.

Fig. 6 4-aminoantipyrine degradation efficiency-time curves by different treatment processes
It can be seen from Fig. 6 that the degradation efficiency of EC is very low. After 120 min EC treatment, only about 3% of 4-aminoantipyrine is degraded. In comparison, DP process is more efficient and the degrdation efficiency reaches 48.0% after 120 min. When 4-aminoantipyrine is treated with PC process for 120 min, the degrdation efficiency is 50.8%. The higher degrdation efficiency of PC than DP indicates the photocatalytic activity of TiO2/ITO film. For PEC process, the degradation efficiency reaches 62.1% after 120 min treatment, obviously higher than the sum of degrdation efficiencies of EC and PC, demonstrating the synergetic effect of photocatalysis and electrolysis. This result is attributed to the effective inhibition of anodic bias potential to the recombination of photogenerated electron-hole pairs which provides more photogenerated electrons and holes to participate the degradation reactions.
3.4 Influence of experimental conditions on PEC degdradation of 4-aminoantipyrine
Figure 7(a) shows the PEC degradation curves of 4-aminoantipyrine by applying different anodic bias potentials. It can be seen that when the bias potential is increased from +0.4 V to +0.8 V, the degradation efficiency is obviously improved. This phenomenon can be attributed to the fact that increasing bias potential could promote the electron transfer rate which improves the inhibition efficiency to the recombination of photogenerated electron-hole pairs. However, while the bias potential is further increased from +0.8 V to +1.0 V, the degradation efficiency is decreased. It is considered that the thickness of the photoanode is finite and thus the thickness of the space charge layer can not exceed the thickness of semicondictive film. Under a fixed light intensity, the photogenerated electrons is also limited. When the bias potential is increased to a value, the photogenerated carrier is sufficiently separated and thus foms the saturated photocurrent [21]. Even if the bias potential is further increased, the photocurrent would not increase. On the contrary, the current efficiency is decreased. Thus, +0.8 V is selected as the optimized bias potential for the PEC degradation of 4–aminoantipyrine on the LPD TiO2/ITO film electrode.
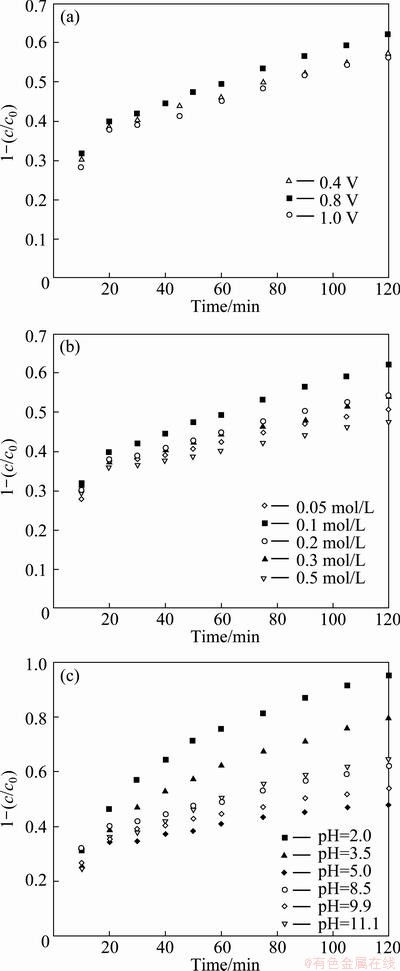
Fig. 7 Effects of bias potential (a), Na2SO4 concentration (b) and pH (c) on degradation efficiency of 4-aminoantipyrine on TiO2/ITO film electrode
The PEC degradation of 4-aminoantipyrine was investigated in the presence of supporting electrolyte Na2SO4 at the concentration of 0.05-0.5 mol/L (Fig. 7(b)). The results indicate that when the concentration of Na2SO4 is increased from 0.05 mol/L to 0.1 mol/L, the degradation efficiency of 4-aminoantipyrine is improved. While the concentration of Na2SO4 is increased to be higher than 0.1 mol/L , the degradation efficiency of 4-aminoantipyrine shows a declining tendency. It is well known that with increasing the concentration of electrolyte concentration, the conductivity of solution is enhanced, which could decrease the cell volatge and improve the applied potential efficiency. While the concentration of Na2SO4 is further increased, more 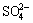 would compete with 4-aminoantipyrine by attracting photogenerated hole, leading to the inhibited degradation. So, 0.1 mol/L was considered the optimized concentration of supporting electrolyte.
would compete with 4-aminoantipyrine by attracting photogenerated hole, leading to the inhibited degradation. So, 0.1 mol/L was considered the optimized concentration of supporting electrolyte.
The pH of solution had an obvious effect on the PEC degradation of 4-aminoantipyrine. Figure 7(c) shows the PEC degradation experimental results in different pH solutions. The pH of solution was adjusted by adding a small amount of H2SO4 or NaOH. It is observed that the highest degradation efficiency is obtained at pH 2.0 and the 120-min degradation efficiency reaches 95.0%, which is obviously higher than that obtained in other pH solutions. The possible explanation is that 4-aminoantipyrine molecule carries more negative charges at pH 2.0, which could be effectively adsorbed on the positvely charged TiO2. While the pH of solution is increased to 5.0, the degradation efficiency is reduced to a low value. Whereas the pH is varied in the alkaline range, the degradation efficiency is not changed regularly. This result implies the complex mechanism for pH effect on the PEC degradation of 4-aminoantipyrine. On one hand, pH influences the band-edge positions of valance and conduction bands of TiO2, surface charge of TiO2, adsorption of organic molecules on the caltalyst surface, as well as the desorption/adsorption of electron-hole pairs. On the other hand, the molecular structure of 4-aminoantipyrine may be changed significantly at high pH, leading to the varied degradation efficiency.
4 Conclusions
1) TiO2 film was deposited on the ITO surface by the LPD process. The obtained TiO2/ITO film had an excellent photoelectrochemical performance.
2) Using such a TiO2/ITO film, the degaration of 4-aminoantipyrine by PEC technique was systematically investigated. Under optimized conditions such as +0.8 V applied bias potential, 0.1 mol/L Na2SO4 and pH 2.0, the highest degradation efficiency could reach 95% for 0.1 mmol/L 4-aminoantipyrine after 120 min PEC treatment.
3) LPD process provides a simple wet-chemical approach to the preparation of TiO2/ITO film. The as-prepared LPD TiO2/ITO film electrode is promising for PEC degradation of organic pollutants.
References
[1] ZHANG Y Z, XIONG X Y, HAN Y, ZHANG X H, SHEN F, DENG S H, XIAO H, YANG X Y, YANG G, PENG H. Photoelectrocatalytic degradation of recalcitrant organic pollutants using TiO2 film electrodes: An overview [J]. Chemosphere, 2012, 88: 145-154.
[2] YASUMORI A, SHINODA H, KAMESHIMA Y, HAYASHI S, OKADA K. Photocatalytic and photoelectrochemical properties of TiO2-based multiple layer thin film prepared by sol-gel and reactive-sputtering methods [J]. Journal of Materials Chemistry, 2001, 11: 1253-1257.
[3] FU Tao, WU Xiao-ming, WU Feng, LUO Meng, DONG Bing-hui, JI Yuan. Surface modification of NiTi alloy by sol-gel derived porous TiO2 film [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(7): 1661-1666.
[4] WANG S, WU X H, QIN W, JIANG Z H. TiO2 films prepared by micro-plasma oxidation method for dye-sensitized solar cell [J]. Electrochimica Acta, 2007, 53(4): 1883-1889.
[5] WANG He-feng, SHU Xue-feng, LI Xiu-yan, TANG Bin. Photocatalytic activities of N doped TiO2 coatings on 316L stainless steel by plasma surface alloying technique [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(s1): s120-s126.
[6] RYU S W,KIM E J,KO S K, HAHN S H. Effect of calcination on the structural and optical properties of M/TiO2 thin films by RF magnetron co-sputtering [J]. Materials Letters, 2004, 58(5): 582-587.
[7] ZHAO Bao-xing, ZHOU Ji-cheng, RONG Lin-yan. Microstructure and optical properties of TiO2 thin films deposited at different oxygen flow rates [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1429-1433.
[8] KWACK W S, MOON H S, JEONG S J, WANG Q M, KWON S H. Hybrid functional IrO2-TiO2 thin film resistor prepared by atomic layer deposition for thermal inkjet printheads [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(s1): s88-s91.
[9] BACKMAM U,AUVINEN A,JOKINIEMI J K. Deposition of nanostructured titania films by particle-assisted MOCVD [J]. Surface and Coatings Technology, 2005, 192(1): 81-87.
[10] HUA X S, ZHANG Y J, MA N H, LI X F, WANG H W. A new coral structure TiO2/Ti film electrode applied to photoelectrocatalytic degradation of reactive brilliant red [J]. Journal of Hazardous Materials, 2009, 172: 256-261.
[11] DEKI S, AOI Y,HIROI O,KAJINAMI A. Titanium(IV) oxide thin films prepared from aqueous solution [J]. Chemistry Letters, 1996, 25(6): 433-434.
[12] YU Qiong-wei, FENG Yu-qi. Application of liquid-phase deposition in analytical chemistry [J]. Progress in Chemistry, 2011, 23(6): 1211-1223. (in Chinese)
[13] HOU Y N, QU J H, ZHAO X, LEI P J, WAN D J, HUANG C P. Electro-photocatalytic degradation of acid orange II using a novel TiO2/ACF photoanode [J]. Science of the Total Environment, 2009, 407: 2431-2439.
[14] DING Y B, YANG C Z, ZHU L H, ZHANG J D. Photoelectrochemical activity of liquid phase deposited TiO2 film for degradation of benzotriazole [J]. Journal of Hazardous Materials, 2010, 175: 96-103.
[15] TANG Yu-lin, GAO Nai-yun, PANG Wei-hai, LI Qing-song. Research on status and removal of pharmaceuticals and personal care products in aquatic environment [J]. Water & Wastewater Engineering, 2008, 34(5): 116-121. (in Chinese)
[16] ONESIOS K M, YU J T, BOUWER E J. Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: A review [J]. Biodegradation, 2009, 20: 441-466.
[17] GRACIA-LOR E, SANCHO J V, SERRANO R, HERNANDEZ F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia [J]. Chemosphere, 2012, 87: 453-462.
[18] HU X Y, YANG J A, YANG C Z, ZHANG J D. UV/H2O2 degradation of 4-aminoantipyrine: A voltammetric study [J]. Chemical Engineering Journal, 2010, 161: 68-72.
[19] ESQUIVEL K, RODRIGUEZ F J, ARRIAGA L G, CAMPS E, DURAN-MORENO A, ESCOBAR-ALARCON L, GODINEZ L A. Photoelectrocatalytic wastewater treatment using TiO2/ITO bilayers prepared on optical fibers by pulsed laser deposition [J]. Journal of Environmental Engineering-ASCE, 2011, 137(5): 355-362.
[20] HAN Song, ZHANG Xing-wang, YU Qing-ni, LEI Le-cheng. Preparation of TiO2/ITO film electrode by AP-MOCVD for photoelectrocatalytic application [J]. Science China: Chemistry, 2012, 55(11): 2462-2470.
[21] LENG Wen-hua, CHENG Shao-an, ZHANG Jian-qing, CAO Chu-nan. Combination degradation of aniline via photoelectrocatalysis and photoge nerating hydrogen peroxide [J]. Acta Scientiae Circumstantiae, 2001, 21(5): 625-627. (in Chinese).
TiO2/ITO膜的液相沉积法制备及其用于4-氨基安替比林降解的光电催化活性
李 丹,童海霞,张 玲
长沙理工大学 化学与生物工程学院,电力与交通材料保护湖南省重点实验室,长沙 410004
摘 要:利用液相沉积(LPD)法在ITO表面制备TiO2薄膜。光电流和交流阻抗(EIS)测试表明,这种TiO2/ITO液相沉积膜具有良好的光电性能,对紫外光产生灵敏、快速的电流响应,在此膜电极上通过施加一定的阳极偏压可有效分离光生电子-空穴对。将TiO2/ITO膜用于4-氨基安替比林的光电催化降解研究,与其他方法相比,利用此液相沉积膜电极的光电催化技术对于4-氨基安替比林的降解具有明显的协同效果,适合于该污染物的降解处理。当外加阳极偏压为+0.8 V,支持电解质Na2SO4浓度为0.1 mol/L,溶液pH为2.0时,0.1 mmol/L 4-氨基安替比林120 min的光电催化降解效率最高可达95%。
关键词:液相沉积;二氧化钛膜电极;光电催化;4-氨基安替比林
(Edited by Hua YANG)
Foundation item: Projects (12JJ3013, 11JJ5010, 10JJ5002) supported by the Natural Science Foundation of Hunan Province, China; Project (2013CL04) supported by the Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, China; Project (2011RS4069) supported by the Planned Science and Technology Program of Hunan Province, China
Corresponding author: Dan LI; Tel: +86-731-85258733; E-mail: ld1004@126.com
DOI: 10.1016/S1003-6326(13)62868-X
Abstract: A thin layer of TiO2 film was deposited on ITO surface via the liquid phase deposition (LPD) process. The photocurrent and electrochemical impedance spectroscopy (EIS) measurements indicated that the as-prepared LPD TiO2/ITO film had an excellent photoelectrochemical performance, which showed a sensitive and rapid response to the UV irradiation. The photogenerated electron-hole pairs could be effectively separated by applying an external bias to the TiO2 film electrode. The LPD TiO2/ITO film was employed to study the photoelectrocatalytic (PEC) degradation of 4-aminoantipyrine. Compared with other techniques, the PEC technique based on such a LPD film electrode had a synergetic effect for 4-aminoantipyrine degradation. When the applied bias potential was +0.8 V and the supporting electrolyte concentration of Na2SO4 was 0.1 mol/L, the highest degradation efficiency within 120 min could reach 95% for 0.1 mmol/L 4-aminoantipyrine solution at pH 2.0.


