
Removal of vanadium from ammonium molybdate solution by ion exchange
LI Qing-gang(李青刚)1, 2, ZHANG Qi-xiu(张启修)2, ZENG Li(曾 理)2,
XIAO Lian-sheng(肖连生)1, 2, YANG Ya-nan(杨亚男)2
1. State Key Laboratory of Metallurgy and Materials of Rare Metal, Central South University,
Changsha 410083, China;
2. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 4 May 2008; accepted 22 October 2008
Abstract:
The separation techniques of vanadium and molybdenum were summarized, and a new method of removal V(Ⅴ) from Mo(Ⅵ) by adsorption with chelate resin was presented. Nine kinds of chelate resins were used to investigate the adsorbent capability of V(Ⅴ) in ammonium molybdate solution with static method. The test results show that DDAS, CUW and CW-2 resins can easily adsorb V(Ⅴ) in ammonium molybdate solution, but hardly adsorb Mo(Ⅵ). The dynamic experimental results show more than 99.5% of V(Ⅴ) can be adsorbed, and the adsorption rate of Mo(Ⅵ) is less than 0.27% at 294-296 K for 60 min at pH 7.42-8.02. The mass ratio of V to Mo decreases to l/5 0000 in the effluent from 1/255 in the initial solution. The loaded resin can be desorbed by 5% NH3?H2O solution, and the vanadium desorption rate can reach 99.6%. The max concentration of vanadium in desorbed solution can reach 20 g/L, while the concentration of molybdenum is less than 0.8 g/L.
Key words:
ammonium molybdenum; vanadium; chelate resin; ion exchange; separation;
1 Introduction
With the ceaseless exploitation of molybdenum resources and the soaring of international molybdenum prices in recent years[1-3], many factories began to extract molybdenum from low grade molybdenum minerals and secondary molybdenum resources, such as Ni-Mo ore (Mo 2%-8%, V 0.3%-2.0%)[4-7], spent catalyst (Mo 2%-12%, V 1%-20%)[8] and fly ash of heavy oil-fired[9-10]. Due to the property similarity of Mo(Ⅵ) and V(Ⅴ) in aqueous solution, it is difficult to separate Mo(Ⅵ) and V(Ⅴ) completely, which results in a vanadium content in the range of 0.05%-0.20% in ammonium molybdate products.
Traditional method of removing vanadium from molybdenum solution is precipitation of ammonium metavanadate [11]. However, this method cannot remove vanadium completely from molybdenum in aqueous solution. LITZ and JOHN[12] invented a separation technique of molybdenum and vanadium by selectively precipitating molybdenum in a form that is substantially free from vanadium. The molybdenum is precipitated in the form of ammonium octamolybdate with a ratio of vanadium to molybdenum about 1/400 in an initial crystallization and a ratio of about ![]() in a subsequent recrystallization, and the vanadium content in ammonium octamolybdate products also reaches 0.05%. SEBENIK et al[13] invented a separation process between molybdenum and vanadium by selectively precipitating molybdenum in acid condition. The molybdenum is precipitated in the form of molybdenum sulfide (MoS3); but it is not appropriate to the solution containing high concentration of molybdenum but low concentration of vanadium. ZHANG et al[14] studied recovery and separation of molybdenum and vanadium from spent catalyst by solvent extraction. The extractant LIX63 was used to selectively extract Mo(Ⅵ) and V(Ⅳ) from sulfuric acid solution containing Mo(Ⅵ), V(Ⅳ), Fe(Ⅲ), Al(Ⅲ), Ni(Ⅱ), Co(Ⅱ), etc, and then vanadium was selectively stripped from organic phase loaded Mo(Ⅵ) and V(Ⅳ) with sulfuric acid. However, this method only can be used for the separation of Mo(Ⅵ) and V(Ⅳ) in solution.
in a subsequent recrystallization, and the vanadium content in ammonium octamolybdate products also reaches 0.05%. SEBENIK et al[13] invented a separation process between molybdenum and vanadium by selectively precipitating molybdenum in acid condition. The molybdenum is precipitated in the form of molybdenum sulfide (MoS3); but it is not appropriate to the solution containing high concentration of molybdenum but low concentration of vanadium. ZHANG et al[14] studied recovery and separation of molybdenum and vanadium from spent catalyst by solvent extraction. The extractant LIX63 was used to selectively extract Mo(Ⅵ) and V(Ⅳ) from sulfuric acid solution containing Mo(Ⅵ), V(Ⅳ), Fe(Ⅲ), Al(Ⅲ), Ni(Ⅱ), Co(Ⅱ), etc, and then vanadium was selectively stripped from organic phase loaded Mo(Ⅵ) and V(Ⅳ) with sulfuric acid. However, this method only can be used for the separation of Mo(Ⅵ) and V(Ⅳ) in solution.
HIRAI et al[15-17] studied reductive stripping of vanadium in solvent extraction process for separation of vanadium and molybdenum. A separation efficiency of 1.64×104 was obtained. HENRY and van LIERDE[18] investigated selective separation of vanadium from molybdenum by electrochemical ion exchange. The principle is that V(Ⅴ) and Mo(Ⅵ) are adsorbed synchronously from neutral solution by Amberlite IRA 94S resin. In the process, V(Ⅴ) is selectively desorbed with molybdenum, then it is reduced to V(Ⅳ) by a cathode mixed with ion exchange resin. The subsequent elution can therefore produce a pure molybdenum solution. The further elution of molybdenum with alkaline solution leads to the recovery of a pure molybdate solution characterized by a molar ratio of V to Mo of 1/l 000 after a post-precipitation at pH 8.
2 Experimental2.1 Materials
Macropore chelate resins DHDS, DHES, DHFS, DD1S, DDAS, DGES and gelatinous chelate resins CW-1, CW-2, CUW were used.
The resin was first soaked for 24 h in pure water, then converted in 1 mol/L H2SO4 or 2 mol/L HCl, and lastly rinsed with pure water before use. The static and dynamic adsorption experiments were carried out. Static adsorption trials were carried out in a beaker containing 2 mL treated resins and 40 mL solution on the magnetic stirring apparatus. After adsorption, the suspension was filtered, and the solution was analyzed for molybdenum and vanadium. Molybdenum was determined with ammonium thiocyanate colorimetry by spectrophotometer. Vanadium was titrated with ammonium ferrous sulfate. The static adsorption rate was determined as follows:
η1=(ρ0-ρ1)/ρ0×100% (1)
where η1 is the static adsorption rate; ρ0 is the concentration of vanadium or molybdenum in initial solution, g/L; ρ1 is the concentration of vanadium or molybdenum in adsorbed solution, g/L.
η2=∑Vi(ρ0-ρi)/(Vρ0)×100% (2)
where η2 is the dynamic adsorption rate; Vi is the volume of the ith effluent sample, L; ρ0 is the concentration of molybdenum or vanadium in initial solution, g/L; ρi is the concentration of the ith effluent sample, g/L; V is the total effluent volume, L.
Bed volume(BV) of effluent means the ratio of effluent volume to filled resin volume in dynamic test.
Work adsorption means the ratio of effluent volume before breakthrough point to filled resin volume in dynamic test.
3 Results and discussion
3.1 Selection of chelate resin
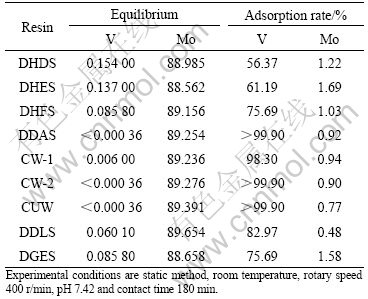
Table 1 lists the experimental results of resins to vanadium. It can be seen from Table 1 that all chelate resins can adsorb vanadium well but hardly adsorb molybdenum. Among these resins, DDAS, CW-2 and CUW have a good adsorption capability and their vanadium adsorption rates all exceed 99.9%, while their molybdenum adsorption rates are only 0.92%, 0.90% and 0.77%, respectively.
Table 1 Selective experimental results of resins to vanadium
3.2 Effect of contact time on adsorption rate of vanadium
In static adsorption, DDAS, CW-2 and CUW resins were used. Experimental conditions were as follows: room temperature, rotary speed 400 r/min, pH 7.42. Experimental results are shown in Fig.1.
Fig.1 shows the relationship between contact time and adsorption rate of vanadium. It can be seen that the adsorptions of CW-2 and CUW are quick, and in about 30 min, the adsorption rate of 98% is obtained. The reaction equilibrium time is about 60 min for CW-2 or CUW, while 80 min for DDAS resin. The reason is that CW-2 and CUW resins are gelatinous resins with small grain size and large specific surface area; DAS resin is macropore resin with large grain size and small specific surface area.
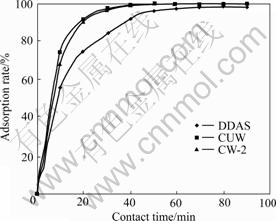
Fig.1 Effect of contact time on adsorption rate of vanadium with different resins
3.3 Comparison of adsorption capabilities of resins
In dynamic adsorption, DDAS, CW-2 and CUW resins were used. Experimental conditions were as follows: room temperature, contact time 60 min and pH 7.42. Experimental results are shown in Fig.2 and Fig.3.
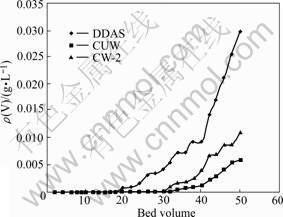
Fig.2 Comparison of adsorption capabilities of resins to vanadium
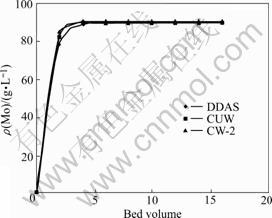
Fig.3 Effluent curves of adsorption of molybdenum with different resins
When the concentration of vanadium in ammonium molybdate solution with 120 g/L Mo was higher than 0.005 g/L, primrose yellow ammonium molybdate crystals precipitated from the solution, and the content of vanadium in crystals reached 0.001 5%. When the concentration of vanadium was lower than 0.003 g/L, the colour of ammonium molybdate crystals precipitated from the solution would be white and the content of vanadium in crystal was below 0.0015%. So, the 0.003 g/L vanadium was chosen as the breakthrough point in dynamic adsorption.
Fig.2 shows that vanadium adsorption capability of CUW resin is the highest up to 44BV, while 38BV for CW-2 resin and 24BV for DDAS resin. The average contents of vanadium in effluent solution with DDAS, CW-2 and CUW were 0.001 8, 0.001 1 and 0.000 73 g/L, respectively, the ratios of vanadium to molybdenum decreased from 1/255 in initial feed to 1/50 000, 1/81818 and 1/123 290, respectively; and vanadium removal rates were 99.49% and 99.69% and 99.79%, respectively.
Fig.3 shows that DDAS, CW-2 and CUW resins can hardly adsorb molybdenum, and the concentration of molybdenum in effluent solution reaches equilibrium rapidly.
3.4 Effect of solution pH on adsorption rate of vanadium
In dynamic test, CUW resin was used. Experimental conditions were as follows: room temperature, and 60 min contact time. Experimental results are shown in Fig.4.
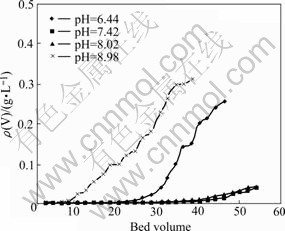
Fig.4 Effluent curves of pH values vs adsorption of vanadium
The adsorption capability was the best in the pH range of 7.42-8.02. When pH of solution was lower than
6.44 or higher than 8.98, the vanadium concentration of ammonium molybdate effused from column was increased rapidly, while the adsorption capability to vanadium was decreased rapidly.
3.5 Desorption and regeneration of loaded resin
Loaded resin with vanadium obtained by previous adsorption tests(section 3.2) was desorbed for 60 min with 5% ammonia liquor. Test results are shown in Fig.5 and Fig.6.
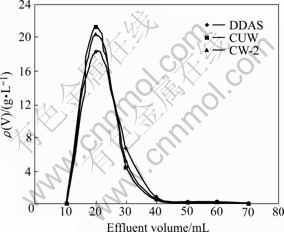
Fig.5 Effluent curves of desorption of vanadium
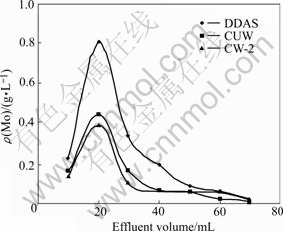
Fig.6 Effluent curves of desorption of molybdenum
It can be seen from Fig.5 and Fig.6 that DDAS, CUW and CW-2 loaded resins can be desorbed completely with 5% ammonia liquor and their vanadium desorption ratios reach 99.61%, 99.83% and 99.78%, respectively. And the peak point of vanadium concentration in desorbed solution is up to 18-22 g/L while that of Mo only is 0.4-0.8 g/L.
During the desorption process, some crystalline white matter appears in column when effluent volume is 10-20 mL. And then the white crystalline matter disappears after effluent volume is above 20 mL. The main reason causing this phenomena is the change of pH value in column. At the beginning of desorption, the pH value reduces, and then the ammonium metavanadate crystal appears at the pH value lower than 9. In the processing of desorption, the pH value of effluent increases gradually and the crystals begin to dissolve again. Reaction equations of desorption process are as follows:
R—VO3+OH-=R—OH+VO3- (3)
VO3-+NH4+=NH4VO3↓ (4)
NH4VO3↓+2NH4OH=VO43-+3NH4++H2O (5)
4 Conclusions
1) Chelate resins can adsorb vanadium easily from ammonium molybdate solution, and DDAS, CUW and CW-2 resins have a higher desorption capacity to vanadium than other chelate resins.
2) Optimization conditions of removing vanadium from molybdenum in ammonium molybdate solution is pH 7.42-8.02, adsorption time more than 60 min.
3) 5% ammonia liquor is used to desorb the loaded resins, the desorption rate of vanadium exceeds 99.6% and the concentration of vanadium in desorption solution is up to 20 g/L.
4) The concentration of vanadium in the ammonia molybdate solution decreases to 0.001 8 g/L from 0.353 g/L. The mass ratio of V to Mo in the ammonium molybdate solution decreases to l/50 000 from 1/255. The content of vanadium in ammonium tetramolybdate production decreases to 0.001 5% from 0.05%-0.1%.
References
[1] YANG Zhao-hui. Market of molybdenum in 2004 [J]. China Molybdenum Industry, 2005, 29(1): 48-53. (in Chinese)
[2] YANG Liu-xiao, XU Jie-yu. Report on the development of molybdenum industry in 2005 [J]. China Molybdenum Industry, 2005, 29(2): 3-6. (in Chinese)
[3] XU Ai-dong, YANG Zhao-hui. Analysis on molybdenum market in recent years [J]. World Nonferrous Metals, 2006(3): 41-46. (in Chinese)
[4] JIANG Shao-yong, YANG Jin-hong, LING Hong-fei, FENG Hong-zhen, CHEN Yong-qun, CHEN Jian-hua. Re-Os isotopes and PGE geochemistry of black shales and intercalated Ni-Mo polymetallic sulfide bed from the Lower Cambrian Niutitang Formation, South China [J]. Progress in Natural Science, 2003, 13(10): 788-794. (in Chinese)
[5] LUO Wei, DAI Ta-gen. Organic metallogeny of the precious metals Ni-Mo-V deposit in black rock series of Lower Cambrian, Northwestern Hunan [J]. Mineral Resources and Geology, 2007, 21(5): 504-509. (in Chinese)
[6] CHEN Jian-hua, PENG Jin-qing, WEN Guan-guo. Preliminary study on occurrence status of molybdenum-vanadium ores of Lower Cambrian Jiumencong Formation, Songtao County, Guizhou [J]. Guizhou Geology, 2007, 2(24): 185-189. (in Chinese)
[7] LI Qing-gang, XIAO Lian-sheng, ZHANG Gui-qing, ZHANG Qi-xiu. Process and practice of ammonium molybdate production from Ni-Mo ore by hydrometallurgy [J]. Chinese Journal of Rare Metals, 2007, 31(s1): 64-69. (in Chinese)
[8] ZHANG Qi-xiu, ZHAO Qin-sheng. Metallurgy of tungsten and molybdenum [M]. Beijing: Metallurgical Industry Press, 2005. (in Chinese)
[9] STAS J, DAHDOUH A, OMAR A. Recovery of vanadium, nickel and molybdenum from fly ash of heavy oil-fired electrical power station [J]. Periodica Polytechnica: Chemical Engineering, 2007, 51(2): 67-70.
[10] WANG Shu-fen, MA Chen-bing, YUAN Ying-bin. Recovery of molybdenum and vanadium from heavy oil desulphurization catalysts [J]. China Molybdenum Industry, 2007, 31(6): 24-26. (in Chinese)
[11] SHI You-fu, WANG Hai-bei. Separation of molybdenum and vanadium from spent catalysts [J]. China Molybdenum Industry, 2004, 28(2): 39-41. (in Chinese)
[12] LIT Z, JOHN E. Process for recovering molybdenum from solution in a form that is substantially free from vanadium: US4814149 [P]. 1989-03-21.
[13] SEBENIK, ROGER F, LA V. Recovery of metal values from spent hydrodesulfurization catalysts: US4495157 [P]. 1985-01-22.
[14] ZHANG W P, INOUE K, YOUSHIZUKA K. Extraction and selective striping of molybdenum[VI] and vanadium[IV] from sulfuric acid solution containing aluminum[III], cobalt[II], nickel[II] and iron[III] by LIX63 in Exxsol D80 [J]. Hydrometallurgy, 1996, 41: 45-53.
[15] TAKAYUKI H, ISAO K. Separation and purification of vanadium and molybdenum by solvent extraction followed by reductive stripping [J]. Journal of Chemical Engineering of Japan, 1990, 23(2): 208-213.
[16] TAKAYUKI H, ISAO K. Electro-reductive stripping of vanadium in solvent extraction process for separation of vanadium and molybdenum using tri-n-octylmethylammonium chloride [J]. Hydrometallurgy, 1993, 33(1/2): 73-82.
[17] TAKAYUKI H, ISAO K. Electro-reductive stripping of vanadium in solvent extraction process for separation of vanadium and molybdenum [J]. Journal of Chemical Engineering of Japan, 1991, 24(1): 124-125.
[18] HENRY P, van LIERDE A. Selective separation of vanadium from molybdenum by electrochemical ion exchange [J]. Hydrometallurgy, 1998, 48: 73-81.
Foundation item: Project(2007AA06Z129) supported by the National High-tech Research and Development Program of China
Corresponding author: LI Qing-gang; Tel/Fax: +86-731-8716206; E-mail: xlshlqg@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60342-8


