Trans. Nonferrous Met. Soc. China 24(2014) 1898-1904
Surface characterization of chalcopyrite interacting with Leptospirillum ferriphilum
Guo-hua GU, Ke-ting HU, Shuang-ke LI
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 31 December 2012; accepted 25 March 2014
Abstract:
The alteration of surface properties of chalcopyrite after biological conditioning with Leptospirillum ferriphilum was studied by adsorption, zeta-potential, contact angle and bioleaching tests. The strains of L. ferriphilum cultured using different energy sources (either soluble ferrous ion or chalcopyrite) were used. The adhesion of bacteria to the chalcopyrite surface was a fast process. Additionally, the adsorption of substrate-grown bacteria was greater and faster than that of liquid-grown ones. The isoelectric point (IEP) of chalcopyrite moved toward that of pure L. ferriphilum after conditioning with bacteria. The chalcopyrite contact angle curves motioned diversely in the culture with or without energy source. The results of X-ray diffraction patterns (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) analysis indicate that the surface of chalcopyrite is covered with sulfur and jarosite during the bioleaching process by L. ferriphilum. Furthermore, EDS results imply that iron phase dissolves preferentially from chalcopyrite surface during bioleaching. The copper extraction is low, resulting from the formation of a passivation layer on the surface of chalcopyrite. The major component of the passivation layer that blocked continuous copper extraction is sulfur instead of jarosite.
Key words:
chalcopyrite; Leptospirillum ferriphilum; surface properties; passivation layer;
1 Introduction
Chalcopyrite (CuFeS2) is the most widespread copper sulfide mineral. Chalcopyrite-containing ore reserves could be exploited through bioleaching; however, the low copper yield and slow dissolution kinetics of chalcopyrite bioleaching by mesophiles have limited its implementation on commercial scale [1]. Consequently, its underlying reaction mechanisms have been the subject of intensive research from chemical and surface analysis [2-4] as well as biochemistry and molecular biology [5,6].
Bacterial adsorption on mineral surface is the initial step in bioleaching [7]. The adhesion of microorganisms to minerals results in alteration of surface chemistry of minerals due to a consequence of the formation of a biofilm on the surface or biocatalysed surface oxidation or reduction products. Therefore, the study on their surface chemical characteristics and the resulting surface properties of minerals are also of critical importance to understand the reaction mechanisms. Our previous results showed that the bacterial mineral interactions change both surface properties, which are related to the bio-oxidation mechanisms [8-10]. Many studies of mineral surface properties before and after interaction with the bacterial cells focus on the flotation and flocculation [11,12]. However, there are few relevant reports in the bio-leaching field.
In this work, the bioleaching actions of Leptospirillum ferriphilum initially grown with different energy sources at the mineral-solution interface are studied. The purpose is to show the change in chalcopyrite surface properties caused by bacterial interaction and to elaborate the mechanism of chalcopyrite bioleaching to some extent.
2 Materials and methods
2.1 Chalcopyrite sample
All bioleaching tests were performed with chalcopyrite mineral from Daye, Hubei province of China. The samples less than 0.074 mm were used for bioleaching experiments. The size less than 5 μm was used for zeta-potential and adsorption tests. The chemical analyses showed that the chalcopyrite contained 30.74% Cu, 30.64% Fe and 33.24% S, and there was a small amount of quartz.
Pure solid chalcopyrite crystals were cut into certain thin sections, and then polished to the exposed faces used for contact angle tests.
2.2 Microorganisms and culture media
For this study, a strain of L. ferriphilum (DQ343299), provided from the Key Laboratory of Biometallurgy in Central South University, was used. Cells of the specie L. ferriphilum were grown aerobically in 9K medium with initial pH 1.6 at 40 °C on a rotary shaker. The 9K medium consisted of 3.0 g/L (NH4)2SO4, 0.1 g/L KCl, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2. Fe2+ ion-grown L. ferriphilum was cultured in 9K medium plus FeSO4·7H2O (44.2 g/L) as energy source. Chalcopyrite-grown L. ferriphilum was cultivated with addition of chalcopyrite. Cells were obtained through filtrating by Whatman filter paper which allowed cells to get through from the suspended solid materials. The suspension-containing cells were successively centrifugation treated (10000g) for 20 min and washed in dilute sulfuric acid (pH 1.6) to eliminate residual ions for experiments.
2.3 Adsorption measurements
Tests were performed at constant mineral-liquid ratio (1:100) in NaCl solution (1 mmol/L) with initial cell concentration of 1×108mL-1. Cells and minerals (<5 μm) were interacted together for 60 min. Then, the bacterial populations remaining in the liquid phase were determined by blood cell counting chambers under a biological microscope.
2.4 Zeta-potential tests
The zeta-potential was measured with the DELSA440SⅡType electrokinetic instrument. The cell concentration was 1×108mL-1 and NaCl solution was used as electrolyte to maintain the ionic strength at 1 mmol/L. The zeta-potential values of chalcopyrite (<5 μm) before and after interacting with bacterial cells were also tested. Minerals were pretreated with cells for 3 h using a magnetic stirrer. All tests were performed as a function of pH adjusted with HCl or NaOH solution.
2.5 Contact angle measurements
The JJC-I wetting angle measurement instrument, produced by Changchun Optics Instrument Factory, China, was used to measure the contact angle of chalcopyrite surface. Polished chalcopyrite coupons were sterilized with 75% ethanol and rinsed with distilled water prior to use. The mineral coupons were hung in flasks under two different environment conditions. Some plates were incubated in 100 mL 9 K medium with L. ferriphilum (1×108 mL-1) only, not adding any energy source. The others were under the condition in accordance with bioleaching environment (the cell concentration of 1×107mL-1, the pulp concentration of 2%) in flasks. In this way, chalcopyrite powders were used as energy source. For all experiments, the test time should be no more than 1 min at the room temperature (about 25 °C).
2.6 Bioleaching tests
All leaching tests were performed in a rotary shaker with a speed of 170 r/min at 40 °C. Experiments were carried out in 250 mL Erlenmeyer flasks with 150 mL solution. A pulp density of 2% (w/v) was chosen, and the cell density was 1×107mL-1. Periodically, water evaporation was restored, pH and redox potential (vs saturated calomel electrode) were recorded and 2 mL sample was removed from the liquid to obtain kinetic information on metal dissolution. Cu concentration was measured by atomic absorption spectrophotometry (AAS).
2.7 Mineral morphology and composition analyses
The leaching residues were filtered and air dried for XRD, scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) tests. All samples were coated with gold prior to SEM and EDS experiments.
XRD was done with an X-ray diffractometer (XRD) (Model D/Max2500PC) with Cu Kα radiation (λ=1.54056 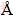 ) in the range of 2θ from 10° to 80°. A scanning electron microscope, JSM-6360LV, was used to determine the chalcopyrite surface changes during bioleaching. In addition, chalcopyrite coupons were also made SEM/EDS analysis.
) in the range of 2θ from 10° to 80°. A scanning electron microscope, JSM-6360LV, was used to determine the chalcopyrite surface changes during bioleaching. In addition, chalcopyrite coupons were also made SEM/EDS analysis.
3 Results and discussion
3.1 Adsorption studies
Bacterial adsorption on the mineral surface as a function of time is shown in Fig. 1. Adsorption to chalcopyrite was a fast process for the different types of L. ferriphilum and the equilibrium was attained after 20 min for chalcopyrite-grown cells, 25 min for Fe2+ ion- grown strain. However, the cell density of L. ferriphilum (88%) grown with minerals on the surface of chalcopyrite was more than that grown with Fe2+ ion (76%). The adsorption of bacteria towards minerals was associated with the cell grown energy source. The adherence of L. ferriphilum grown with chalcopyrite to chalcopyrite was greater and faster than the cells grown with Fe2+ ion. It has been reported [13] that the substrate-grown cells surfaces contain higher protein content than the ferrous ion-grown strains. In other words, substrate-grown cells have a relatively high hydrophobicity than solution-grown cells. Herein, the same strain grown under different conditions may have different adhesion ability toward sulfide minerals.
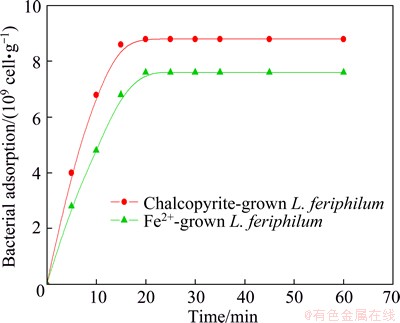
Fig. 1 Adsorption curves of L. ferriphilum grown under different conditions
3.2 Zeta-potential measurements
The strain of L. ferriphilum was acidophil, so zeta-potential tests were conducted in acidic environment (pH<7) except experiment for the pure chalcopyrite.
The results of zeta-potential as a function of pH for L. ferriphilum cultured in different energy substances are presented in Fig. 2. Strain grown with Fe2+ ion in the whole pH range displayed a negative charge and exhibited an iso-electric point (IEP) at below pH 2.0, while the IEP of minerals-grown one was located at around pH 3.6.
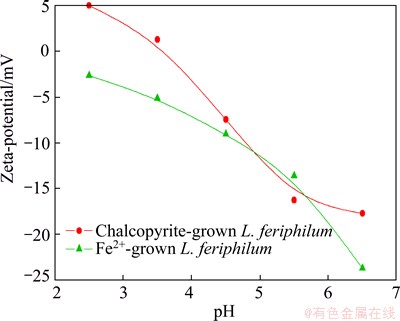
Fig. 2 Zeta-potentials of L. ferriphilum grown under different conditions as function of pH
The zeta-potentials of chalcopyrite before and after interaction with bacteria are illustrated in Fig. 3. The IEP of pure chalcopyrite displayed at about pH 5.7. After interaction with bacteria, the IEP of chalcopyrite moved towards to 4.4 after interacting with Fe2+ ion cultured cells and 3.9 for the other. Furthermore, the movement of the chalcopyrite zeta-potential was consistent with adsorption.
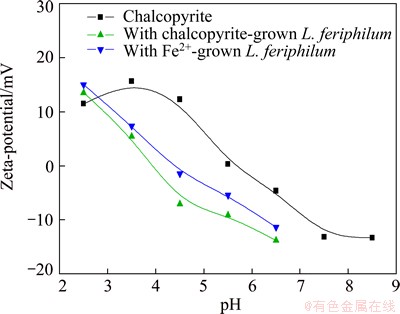
Fig. 3 Zeta-potentials of chalcopyrite before and after interaction with L. ferriphilum grown under different conditions as function of pH
It could be noticed that in acidic medium (pH<2.7), the zeta-potentials of chalcopyrite increased after interaction with L. ferriphilum. This phenomenon can be explained by the adsorption of dissolved Fe3+ from bacterial oxidation of mineral. Although there are various statements about the reactions on the surface, it can be proposed that the related reactions of the oxidation of chalcopyrite are as follows:
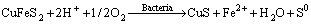 (1)
(1)
 (2)
(2)
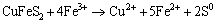 (3)
(3)
3.3 Contact angle measurements
Data from contact angle of chalcopyrite coupons leached without energy source are presented in Fig. 4. The contact angle changes were similar for samples treated by two types of L. ferriphilum. During the test, no product was observed on the surface of chalcopyrite coupons. The contact angle of the chalcopyrite decreased during the whole process, which meant that surface hydrophilicity of the mineral increased and also proved the specific adsorption of cells on the mineral surfaces. The medium without energy source (Fe2+ ion or chalcopyrite) cannot support the growth of L. ferriphilum. During 7 d test, the contact angles for both of the samples decreased slightly, resulting from the attachment of cells to the chalcopyrite. The attached cells made the mineral surface become hydrophilic due to the exposed surface of cells away from the mineral surface [11,14].
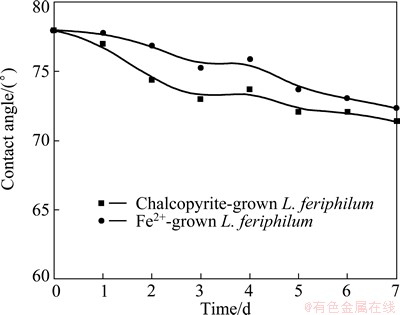
Fig. 4 Changes of contact angle of chalcopyrite in culture without energy source
Figure 5 shows the change in chalcopyrite contact angle in bioleaching condition. On the contrary, the contact angle kinetics was quite different from contact angle mentioned above in the solution without mineral powders.
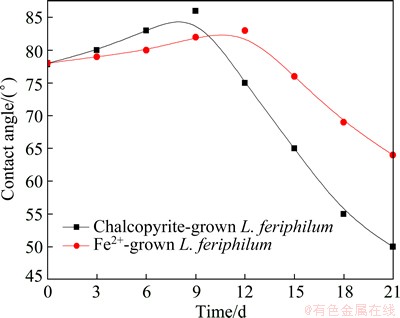
Fig. 5 Changes of chalcopyrite contact angle in culture with chalcopyrite powders as energy source
The increased contact angles of chalcopyrite surface at initial bioleaching stage may result from the formation of elemental sulfur and intermediate copper sulphides on chalcopyrite surface (Eq. (1)). After a period of bioleaching, the contact angles of chalcopyrite surface decreased, which inferred vast formation of jarosite precipitation, a hydrophilic product formed (Eq. (4)). Additionally, chalcopyrite coupons bioleached by minerals-grown L. ferriphilum were investigated by SEM/EDS analysis in the following context, which confirmed the generation of these reaction products.
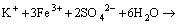
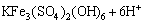 (4)
(4)
3.4 Chalcopyrite bioleaching
Copper ion content, redox potential and pH values as functions of time leached by different types of L. ferriphilum are shown in Figs. 6 and 7, respectively. In Fig. 6, copper extraction increased quickly from the beginning to the 9th day for chalcopyrite-grown bacteria and to the 15th day for Fe2+ ion-grown bacteria, where the copper concentrations in solution were 1.68 g/L and 1.72 g/L, respectively. Thereafter, the chalcopyrite dissolution rate became very slow and after the 21th day the copper concentrations were 2.2 g/L and 2.02 g/L, which corresponded to 35.90% and 32.96% copper extraction, respectively. Obviously, the leaching rate was low and the dissolution of chalcopyrite was not complete. Moreover, during the whole bioleaching process, L. ferriphilum cultured by minerals exhibited a favorable effect on the leaching yields.
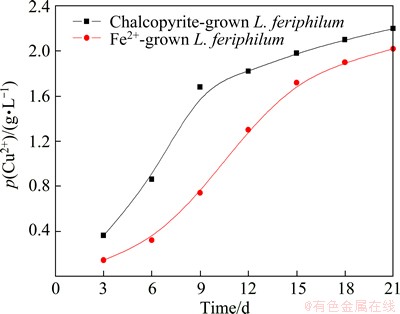
Fig. 6 Copper ion concentration as function of time during bioleaching of chalcopyrite by L. ferriphilum grown with different energies
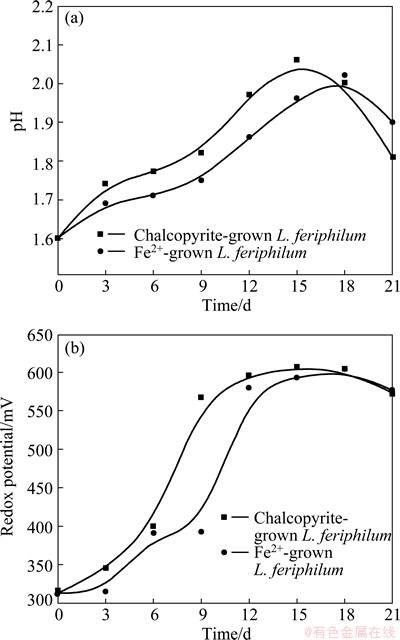
Fig. 7 pH (a) and redox potential (b) as function of time during bioleaching
The pH values of the solutions in the bioleaching by two types of L. ferriphilum were nearly the same, with the two curves overlapping and following each other, so was the case of redox potential. The pH value increased rapidly at the initial stage and reached about 2.0 after 15-18 d, and then the pH declined, demonstrating that the leaching of chalcopyrite by L. ferriphilum was an acid consuming process at the early stage of bioleaching and an acid production process later on. The redox potentials of the solutions reached a plateau after about 9 d and 15 d for the exposure to chalcopyrite-grown strain and Fe2+ ion-grown strain, respectively. Obviously, the change was faster in the solution with the chalcopyrite- grown bacteria culture.
3.5 XRD and SEM/EDS analysis
The XRD patterns for chalcopyrite residues, which have interacted with Fe2+-grown and chalcopyrite-grown L. ferriphilum after 8 d and 21 d, respectively, are shown in Fig. 8.
Sulfur was observed after being leached for 8 d by chalcopyrite-grown L. ferriphilum. By contrast, no product was discovered on the surface of residues conducted with the Fe2+-grown cells. At the end of bioleaching, a lot of jarosite and sulfur existed in the residues after bioleaching with both two types of L. ferriphilum.
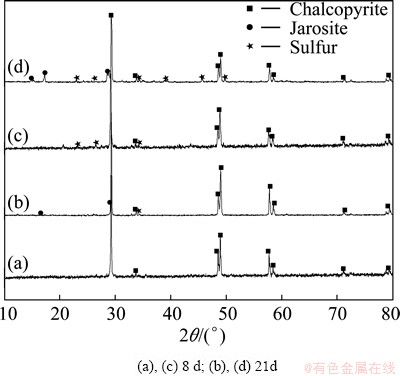
Fig. 8 XRD patterns for leached chalcopyrite residues by Fe2+-grown L. ferriphilum (a, b) and chalcopyrite-grown L. ferriphilum (c, d) at different time
Figure 9 shows the SEM images and EDS analysis for the residues leached by chalcopyrite-grown L. ferriphilum. Before leaching, the mass fractions of elements in chalcopyrite were 30.74% Cu, 30.64% Fe and 33.24% S and the mole ratio of Cu to Fe to S was 1:1.14:2.16, which were very close to the stoicheometry of chalcopyrite. After leaching by L. ferriphilum for 8 d, the surface of chalcopyrite was smooth and tight from SEM image. At that time, the mass fractions of elements were 32.92% Cu, 28.94% Fe and 38.13% S and the mole ratio of Cu to Fe to S was 1:1:2.30 due to the formation of sulfur on the chalcopyrite surface. Obviously, the amount of element Fe on the surface of chalcopyrite decreased. It has been reported that the release of iron into electrolyte or oxyhydroxide phases is usually faster than that of copper, so the formation of copper sulfides, first of all covellite, CuS, and unstable or metastable passive layers of Cu1-xS or Cu1-xFe1-yS2 composition was assumed [15].
At the end of leaching by L. ferriphilum, a lot of loose precipitations could be observed from the SEM image, some of which sticked together and fell off from chalcopyrite surface. The mass fractions of elements were 9.09% Cu, 53.02% Fe and 29.26% S, and the mole ratio of Cu to Fe to S was 1:6.63:6.38, which indicated that a large amount of jarosite was generated and sulfur was constantly enriched. Sulfur and jarosite formed during bioleaching of chalcopyrite, which could illustrate the changes of pH value during bioleaching by L. ferriphilum.
Figure 10 further shows the SEM and EDS analysis results of chalcopyrite coupons conducted by mineral- grown L. ferriphilum after 8 and 21 d during the contact angle experiments. Evidence was observed for the formation of a small number of tight sulfur after 8 d. Meanwhile, large quantities of iron oxide phase and sulfur phase have been deposited on lump surface in the end. The feature of precipitation on the surface of chalcopyrite plate (see enlarged image in the upper-right corner) was the same as that on the leached residues, further indicating that the same loose products occurred.

Fig. 9 SEM images (a, b) and EDS analyses (a′, b′) of leached residues by chalcopyrite-grown L. ferriphilum
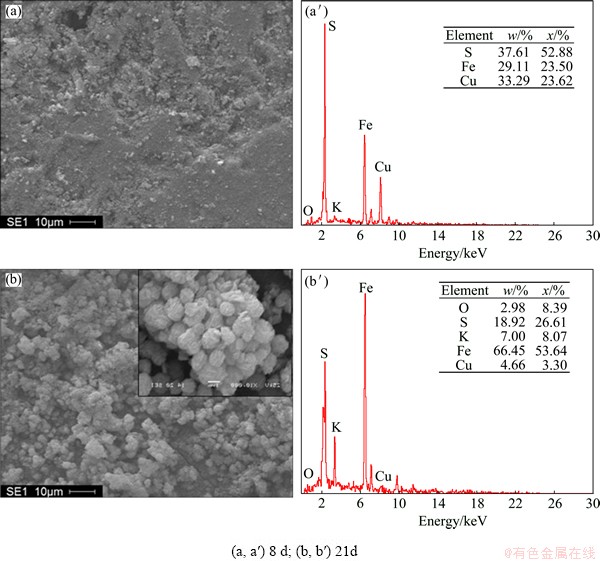
Fig. 10 SEM images (a, b) and EDS analyses (a′, b′) of leached coupons conducted by mineral-grown L. ferriphilum during contact angle tests
From XRD and SEM/EDS analyses of chalcopyrite residues and coupons, sulfur and jarosite precipitations are formed during the leaching process. At the initial stage of bioleaching by chalcopyrite-grown bacteria, the product sulfur was smooth and tight, indicating that it may be the reason of chalcopyrite passivation. It was consistent with copper extraction (Fig. 6) that the rate of leaching was declined from about 9 d. Moreover, at this time, no sulfur was discovered on the surface of residues after conducting with Fe2+-grown L. ferriphilum, so the rate of leaching increased without block. In the later period, a large amount of loose jarosite formed, which would not be an effective layer to inhibit the diffusion of microorganisms, nutrients and reaction products from the mineral surface. Therefore, a tight passivation layer mainly containing sulfur on the mineral surface may block the continuous copper extraction during bioleaching. Though the amount of jarosite was huge in the end, it was loose and started to fall off from chalcopyrite surface, which cannot cause passivation.
5 Conclusions
1) The contact angle curves motion diversely in the culture with or without energy source: the surface of chalcopyrite displays a property of bacterial adsorption, while the other presents evolution of surface components.
2) Growth substrate has a close relationship between the surface properties of bacteria and their interaction behavior with chalcopyrite. Chalcopyrite- grown bacteria have a stronger affinity to chalcopyrite compared with the Fe2+ ion-grown bacteria.
3) During chalcopyrite bioleaching by L. ferriphilum, sulfur forms at the early stage and enriches on the surface of minerals in the whole process, then loose jarosite generates late in the bioleaching. The low copper extraction rate at the final stage of bioleaching is due to the passivation of the chalcopyrite surface, which inhibits the electrochemical transport between the bioleaching solution and the mineral surface. The component of the passivation layer is sulfur rather than jarosite.
References
[1] GU Guo-hua, GUO Yu-wu. Chalcopyrite dissolution behavior under microbe-mineral contact/uncontact model [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 2167-2172. (in Chinese)
[2] KLAUBER C. Fracture-induced reconstruction of a chalcopyrite (CuFeS2) surface [J]. Surf Interface Anal, 2003, 35: 415-428.
[3] SASAKI K, NAKAMUTA Y, HIRAJIMA T, TUOVINEN O H. Raman characterization of secondary minerals formed during chalcopyrite leaching with Acidithiobacillus ferrooxidans [J]. Hydrometallurgy, 2009, 95: 153-158.
[4] XIA J, YANG Y, HE H, LIANG C, ZHAO X, ZHENG L, MA C, ZHAO Y, NIE Z, QIU G. Investigation of the sulfur speciation during chalcopyrite leaching by moderate thermophile Sulfobacillus thermosulfidooxidans [J]. Int J Miner Process, 2010, 94: 52-57.
[5] BARRETO M, JEDLICKI E, HOLMES D S. Identification of a gene cluster for the formation of extracellular polysaccharide precursors in the chemolithoautotroph Acidithiobacillus ferrooxidans [J]. Appl Environ Microb, 2005, 71: 2902-2909.
[6] SAND W, GEHRKE T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria [J]. Res Microbiol, 2006, 157: 49-56.
[7] BRIERLEY C L. Microbiological mining [J]. Scientific American, 1982, 247: 42-53.
[8] GU G, SU L, CHEN M, SUN X, ZHOU H. Bio-leaching effects of Leptospirillum ferriphilum on the surface chemical properties of pyrite [J]. Mining Science and Technology, 2010, 20: 286-291.
[9] GU G, ZHAO K, QIU G, HU Y, SUN X. Effects of Leptospirillum ferriphilum and Acidithiobacillus caldus on surface properties of pyrrhotite [J]. Hydrometallurgy, 2009, 100: 72-75.
[10] CHEN Ming-lian, ZHANG Lin, GU Guo-hua, HU Yue-hua, SU Li-jun. Effects of microorganisms on surface properties of chalcopyrite and bioleaching [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1421-1426.
[11] CHANDRAPRABHA M N, NATARAJAN K A. Surface chemical and flotation behaviour of chalcopyrite and pyrite in the presence of Acidithiobacillus thiooxidans [J]. Hydrometallurgy, 2006, 83: 146-152.
[12] SHARMA P K, DAS A, HANUMANTHA RAO K, FORSSBERG K S E. Thiobacillus ferrooxidans interaction with sulphide minerals and selective chalcopyrite flotation from pyrite [C]//SEM Annual Meeting, Advances in Flotation Technology. Denver, 1999: 147-165.
[13] SHARMA P. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions [J]. Hydrometallurgy, 2003, 71: 285-292.
[14] DEVASIA P, NATARAJAN K A. Adhesion of Acidithiobacillus ferrooxidans to mineral surfaces [J]. Int J Miner Process, 2010, 94: 135-139.
[15] FARQUHAR M. Electrochemical oxidation of the chalcopyrite surface: An XPS and AFM study in solution at pH 4 [J]. Appl Surf Sci, 2003, 218: 34-43.
嗜铁钩端螺旋菌对黄铜矿表面性质的影响
顾帼华,胡可婷,李双棵
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:通过吸附、动电位、接触角和摇瓶浸出试验研究Leptospirillum ferriphilum菌作用前后黄铜矿表面性质的变化。采用不同能源物质(亚铁和黄铜矿粉)培养L. ferriphilum菌。结果表明,细菌可以很快吸附在黄铜矿表面,并且固体能源物质培养的细菌比液体能源物质培养的细菌可以更多、更快地吸附在矿物表面。与细菌作用后,黄铜矿的等电点朝着细菌等电点的方向移动。在添加与不添加能源物质时,黄铜矿的接触角表现出不同的变化趋势。XRD、SEM/EDS检测表明浸出过程中在黄铜矿表面生成了硫和黄钾铁矾。通过EDS检测可知在黄铜矿的分解过程中,铁优先从黄铜矿表面释放出来。在浸出过程中黄铜矿表面生成了钝化层,从而导致其浸出率很低。通过研究推测钝化层的主要成分是硫,而不是黄钾铁矾。
关键词:黄铜矿;嗜铁钩端螺旋菌;表面性质;钝化层
(Edited by Xiang-qun LI)
Foundation item: Project (2010CB630903) supported by the National Basic Research Program of China
Corresponding author: Ke-ting HU; Tel: +86-731-88830545; E-mail: huketing0401@163.com
DOI: 10.1016/S1003-6326(14)63269-6
Abstract: The alteration of surface properties of chalcopyrite after biological conditioning with Leptospirillum ferriphilum was studied by adsorption, zeta-potential, contact angle and bioleaching tests. The strains of L. ferriphilum cultured using different energy sources (either soluble ferrous ion or chalcopyrite) were used. The adhesion of bacteria to the chalcopyrite surface was a fast process. Additionally, the adsorption of substrate-grown bacteria was greater and faster than that of liquid-grown ones. The isoelectric point (IEP) of chalcopyrite moved toward that of pure L. ferriphilum after conditioning with bacteria. The chalcopyrite contact angle curves motioned diversely in the culture with or without energy source. The results of X-ray diffraction patterns (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) analysis indicate that the surface of chalcopyrite is covered with sulfur and jarosite during the bioleaching process by L. ferriphilum. Furthermore, EDS results imply that iron phase dissolves preferentially from chalcopyrite surface during bioleaching. The copper extraction is low, resulting from the formation of a passivation layer on the surface of chalcopyrite. The major component of the passivation layer that blocked continuous copper extraction is sulfur instead of jarosite.


