网络首发时间: 2016-06-30 09:04
不同水性粘结剂在LiFePO_4电极中的应用
北京有色金属研究总院国联汽车动力电池研究院有限责任公司
摘 要:
采用常见商业水性粘结剂CMC(羧甲基纤维素钠)、SBR(丁苯橡胶)、LA133、Alg(海藻酸钠)和自主研发的磷酸铁锂材料制备了电极,并采用扫描电镜(SEM)、恒流充放电、X射线衍射(XRD)等测试方法考察了不同水性粘结剂制备的磷酸铁锂电极的微观形貌、电化学性能及循环前后电极材料的结构变化。实验结果表明,粘结剂溶液的粘度、粘结剂的柔韧性及粘结剂在锂离子电池中的电化学稳定性等均对所制备电极的电化学性能影响较大。相比而言,在2.0~4.2 V的电压范围内,由CMC/SBR(4∶6)复合粘结剂制备的磷酸铁锂电极具有最优的电化学性能。在34 m A·g~(-1)(0.2C)的电流密度下,该电极的首周放电比容量为~163.8 m Ah·g~(-1),首次库伦效率为96.8%;在85 m A·g~(-1)(0.5C)的电流密度下该电极的放电比容量约为156 m Ah·g~(-1)且恒流充放150周后电极的容量保持率为97%;当电流密度高达850 m A·g~(-1)(5.0C)时,电极仍可发挥出~115 m Ah·g~(-1)的放电比容量,表现出较好的应用前景。
关键词:
中图分类号: TQ131.11;TM912
作者简介:张海燕(1988-),女,河南南阳人,博士研究生,研究方向:锂离子电池;E-mail:zhy_infanta@163.com;;卢世刚,教授;电话:010-82241193;E-mail:lusg8867@163.com;
收稿日期:2015-05-25
基金:国家自然科学基金项目(51202014);北京市科技计划项目(Z151100000115015)资助;
Application of Different Water-Based Binders in LiFePO_4 Electrode
Zhang Haiyan Pang Jing Lu Shigang
China Automotive Battery Research Institute,General Research Institute for Nonferrous Metals
Abstract:
Lithium iron phosphate positive electrodes were prepared with different commercial water-based binders,such as CMC( sodium carboxymethyl cellulose),SBR( styrene-butadiene rubber),LA133 and Alg( sodium alginate). The morphologies,electrochemical performance and crystalline structure changes of the as-prepared electrodes were all investigated by scanning electron microscope( SEM),galvanostatic charge-discharge tests and X-ray diffraction( XRD). The experimental results demonstrated that the viscosity of the glue,the tenacity and electrochemical stability of binders in lithium-ion battery environment all had a big influence on the electrochemical performance of the prepared electrodes. Compared with others,lithium iron phosphate positive electrodes prepared with CMC and SBR composite binders showed a better electrochemical performance in the voltage range of 2. 0 ~ 4. 2 V. At a current density of 34 mA ·g~(-1)( 0. 2C),the first discharge specific capacity of the electrode was ~ 163. 8 mA h·g~(-1)and its first coulombic efficiency was 96. 8%. At a current density of 85 mA ·g~(-1)( 0. 5C),the discharge specific capacity of this electrode was ~ 156 mA h·g~(-1),and its capacity retention was about 97% in 150 cycles. At the current density of as high as 850 mA ·g~(-1)( 5. 0C),the electrode could still deliver a specific capacity of ~ 115 mA h·g~(-1),suggesting an excellent potential application.
Keyword:
water-based binder; lithium battery; lithium iron phosphate(LiFePO4);
Received: 2015-05-25
目前,在锂离子电池规模化生产中,各企业多以聚偏氟乙烯(PVDF)为粘结剂
磷酸铁锂正极材料因具有结构稳定、循环性能好、安全、无污染且价格便宜等优点,在新能源汽车和大规模储能锂离子电池领域有很好的应用前景
本文考察了几种常用水性粘结剂在自主研发的Li Fe PO4电极材料中的应用性能,对比了它们的制备工艺、循环和倍率性能等,为后期水性粘结剂在Li Fe PO4电极材料中的工业化应用打下基础。
1 实验
1.1 材料
实验所用正极材料为Li Fe PO4(北京有色金属研究总院,电池级),导电剂为导电炭黑Super P(特米高石墨有限公司,电池级),粘结剂分别为CMC(国药集团化学试剂有限公司,分析纯,Mw(重均分子量):~1000000,粘度:30000 m Pa·s(固含量2%))、SBR(JSR公司,电池级,粘度:51m Pa·s(固含量50%))、Alg(Sigma-Aldrich,Mw:800000~1200000,G/M=1.56,粘度:1600 m Pa·s(固含量2%))、LA133(成都茵地乐电源科技有限公司,电池级,粘度:7300 m Pa·s(固含量15%),使用时固含量稀释为5%)。
1.2 电极制备与电池组装
按质量比8∶1∶1称量Li Fe PO4正极材料、导电炭黑Super P和粘结剂,先将电极材料与导电炭黑研磨混合均匀,然后按比例加入粘结剂制成浆料并涂覆在20μm厚的铝箔(住友轻金属有限公司,电池级)上;以LA133和Alg为粘结剂制备的电极在室温条件下干燥并碾压后在80℃下干燥4 h;由CMC/SBR(4∶6)混合粘结剂制备的电极可直接在80℃下干燥。将干燥后的极片冲切为Φ14 mm的圆片,称量并碾压控制极片压实密度为~2.0 g·cm-3,将制备好的极片在80℃下真空干燥12 h备用。
以Li Fe PO4极片为正极,金属锂片(天津中能锂业有限公司,电池级)为负极,Celgard 2500膜(美国Celgard公司)为隔膜,1 mol·L-1Li PF6/EC+DMC+EMC(体积比1∶1∶1,国泰华荣化工新材料有限公司)为电解液,在充满氩气的手套箱中组装CR2032扣式半电池。
1.3 性能测试
用S-4800型扫描电镜(日本Hitachi公司)观察由不同粘结剂制备的电极的微观形貌;用X'Pert PRO型X射线衍射仪(荷兰PANalytical公司)检测电极材料的结构。用CHI1140电化学工作站进行线性扫描伏安测试,电位范围为OCV-4.2 V(vs Li+/Li),扫速为1 m V·s-1。在CT2001A电池测试系统(武汉市金诺电子有限公司)上进行恒流充放电测试,电压范围为2.0~4.2 V,1.0C为170 m A·g-1;循环性能测试:0.5C恒流充电,0.5C恒流放电;倍率性能测试:0.5C恒流充电,分别以0.5,1.0,2.0,3.0和5.0C恒流放电,每个倍率下循环10周。
2 结果与讨论
2.1 粘结剂的结构和性质
几种粘结剂的结构如图1所示,从图1中可以看出CMC(羧甲基纤维素钠)是一种高分子纤维素醚,SBR(丁苯橡胶)是丁二烯和苯乙烯无规共聚得到的高分子聚合物。实验结果显示,CMC的水溶液粘稠、稳定性好,但单独使用制备的极片很脆,难以涂布;SBR是一种高弹性材料,其水乳液为小分子线性链状乳液,粘结力强但乳液粘度较小、不易分散;CMC和SBR一定比例混合使用可以互相弥补缺陷从而得到非常好的涂布性能,其中CMC主要起增稠作用。LA系列水性粘结剂是主链段为聚乙腈(PAN)单一共聚物的水分散液,粘度小,且由于-CN基团极性较强,分子间作用力很大,因此制得的极片较硬、柔韧性偏差。Alg(海藻酸钠)是由a-L-甘露糖醛酸(M单元)与b-D-古罗糖醛酸(G单元)依靠1,4-糖苷键随机连接组成的共聚物,是一种高粘性的高分子化合物,亲水性强,在水中溶解性好,多为中性,单独作为粘结剂使用即具有较好的涂布性能且制备的极片具有一定的韧性。
图1 不同粘结剂的结构Fig.1 Structures of different water-based binders
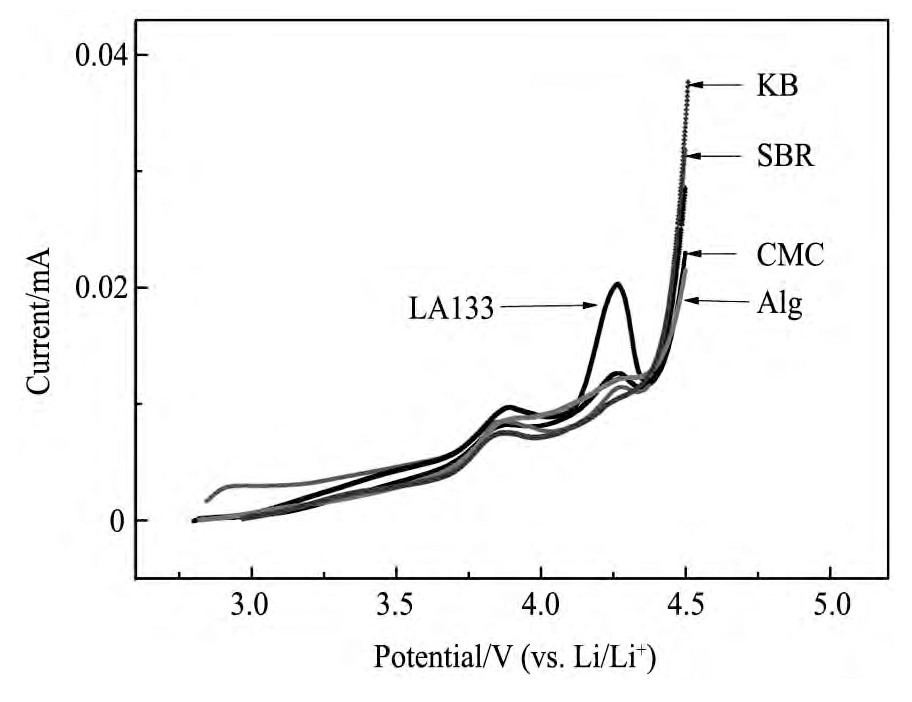
为考察几种粘结剂的电化学稳定性,对比了它们在锂离子电池环境中的伏安曲线,结果如图2所示。从图2可以看出,在开路电位至4.4 V的电位范围内,除粘结剂LA133外其余各粘结剂的阳极极化曲线与空白电极(KB)基本一致,无明显氧化峰出现,表现出较好的电化学稳定性;而由于粘结剂LA133的存储性能较差,长期放置后稳定性下降,实验所用LA133粘结剂在4.25 V出现一微弱的氧化峰,可能会对电极的电化学性能产生一定影响。
图2 不同粘结剂的氧化稳定性Fig.2 Oxidation stabilities of different water-based binders
2.2 SEM分析
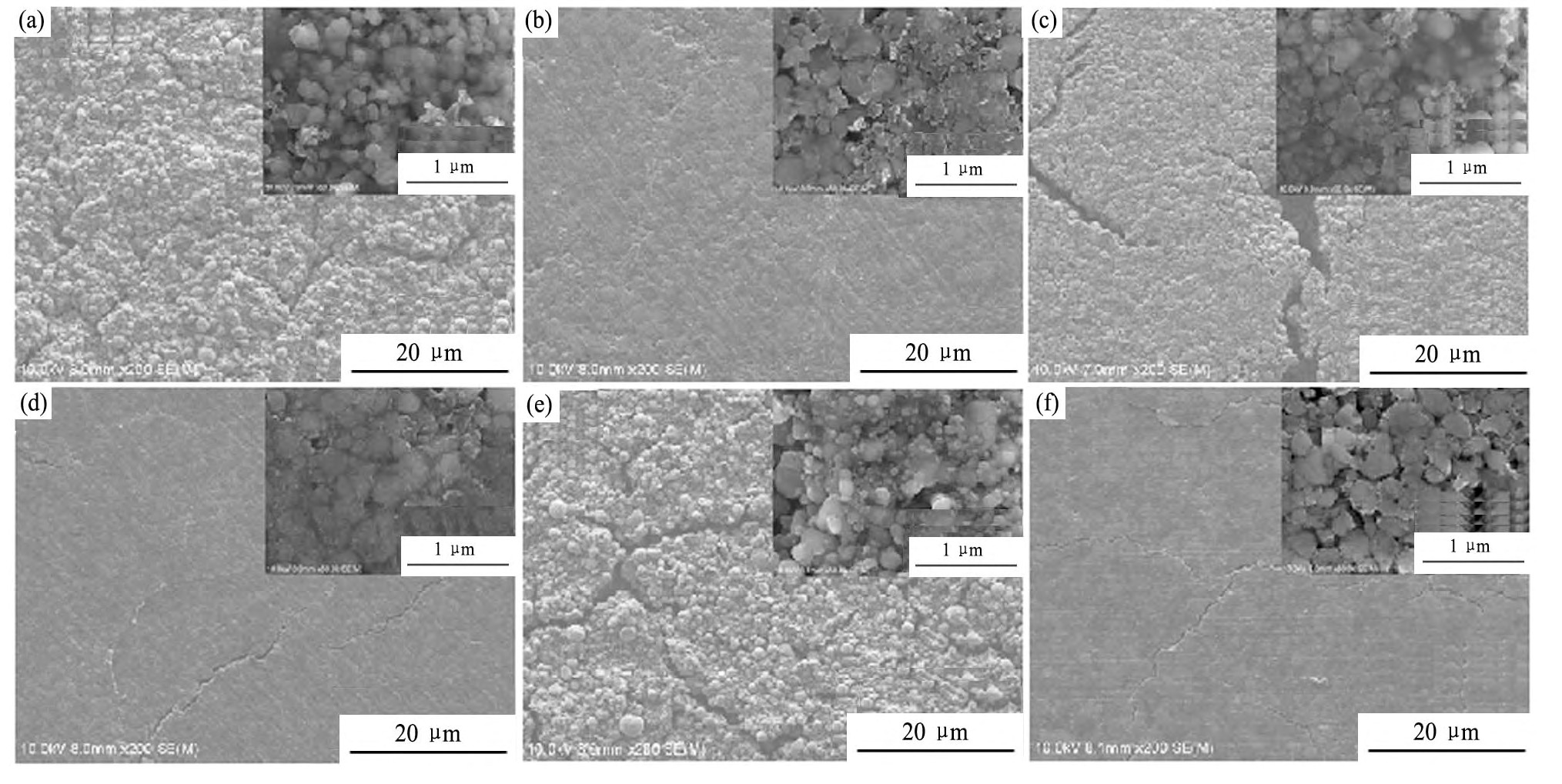
不同水性粘结剂制备的Li Fe PO4电极的微观形貌见图3。对比图3(a~c)(循环前)、(d~f)(循环后)可以发现,即使是在80℃下快速干燥,由CMC/SBR复合型粘结剂制备的电极极片仍具有优异的机械性能且表面裂纹较小;而以LA133为粘结剂制备的电极柔韧性最差,降低干燥温度仍不能有效缓解其在干燥过程中表面龟裂的现象。此外,对比图3(a~c)局部放大图可以看出以CMC/SBR和Alg为粘结剂制备的电极浆料分散效果更优,极片中无明显大颗粒,主要原因可能为水溶液的表面张力较大,溶剂不易浸润电极材料并进行分散,因此,在机械搅拌过程中粘度较大的CMC/SBR,Alg粘结剂与电极材料相互作用力更强,分散效果更好。由以上结果可以看出,粘结剂柔韧性及其水溶液的粘度对制得的电极的性能影响很大,对比而言,CMC/SBR复合型粘结剂的应用性能最好。
2.3 电化学性能测试
2.3.1 充放电性能
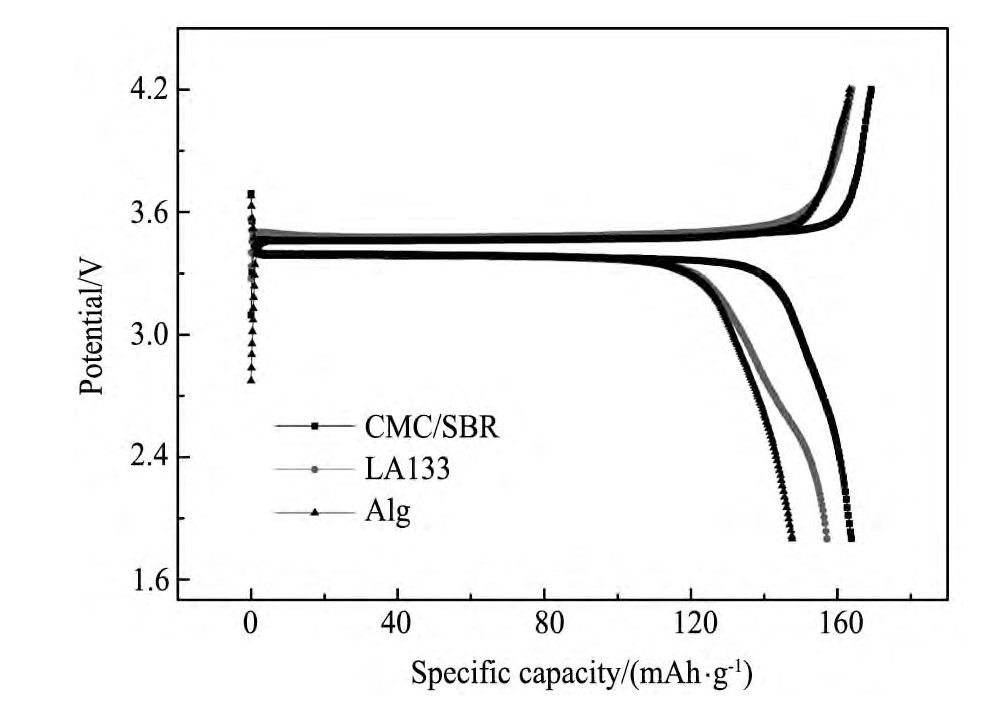
由不同水性粘结剂制备的Li Fe PO4电极的首周充放电曲线见图4,首周充放电容量及效率见表1。从图4可以看出,不同水性粘结剂制得的Li Fe PO4电极充电平台均在~3.47V,放电平台在~3.38 V,与Li Fe PO4电极材料的充放电特征相符;其中,以CMC/SBR混合粘结剂制备的Li Fe PO4电极在电流密度为34 m A·g-1(0.2C)时,首次充电容量为169.3 m Ah·g-1,接近Li Fe PO4电极材料的理论比容量170 m Ah·g-1,放电容量为163.8 m Ah·g-1,其首次充放电效率高达96.8%,明显优于由另外两种粘结剂制备的电极。
图3 不同粘结剂制备的Li Fe PO4电极循环前后的SEM照片Fig.3 SEM images of Li Fe PO4electrodes prepared with different water-based binders before(a~c)and after(d~f)cycling
(a,d)CMC/SBR;(b,e)LA133;(c,f)Alg
图4 不同水性粘结剂制备的Li Fe PO4电极在0.2C下的首次充放电曲线Fig.4 Initial charge-discharge curves of Li Fe PO4electrodes prepared with different water-based binders at a current density of 0.2C
表1 不同水性粘结剂制备的Li Fe PO4电极的首次充、放电容量和效率Table 1 Initial charge-discharge capacity and efficiency of Li Fe PO4electrodes prepared with different wa-ter-based binders 下载原图
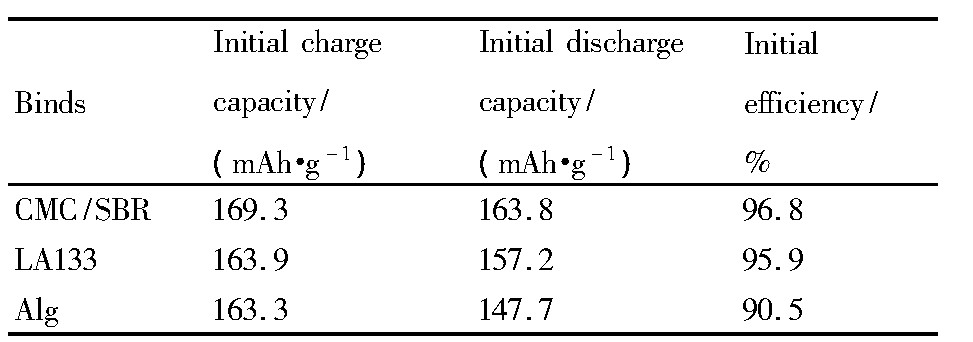
表1 不同水性粘结剂制备的Li Fe PO4电极的首次充、放电容量和效率Table 1 Initial charge-discharge capacity and efficiency of Li Fe PO4electrodes prepared with different wa-ter-based binders
2.3.2 循环性能
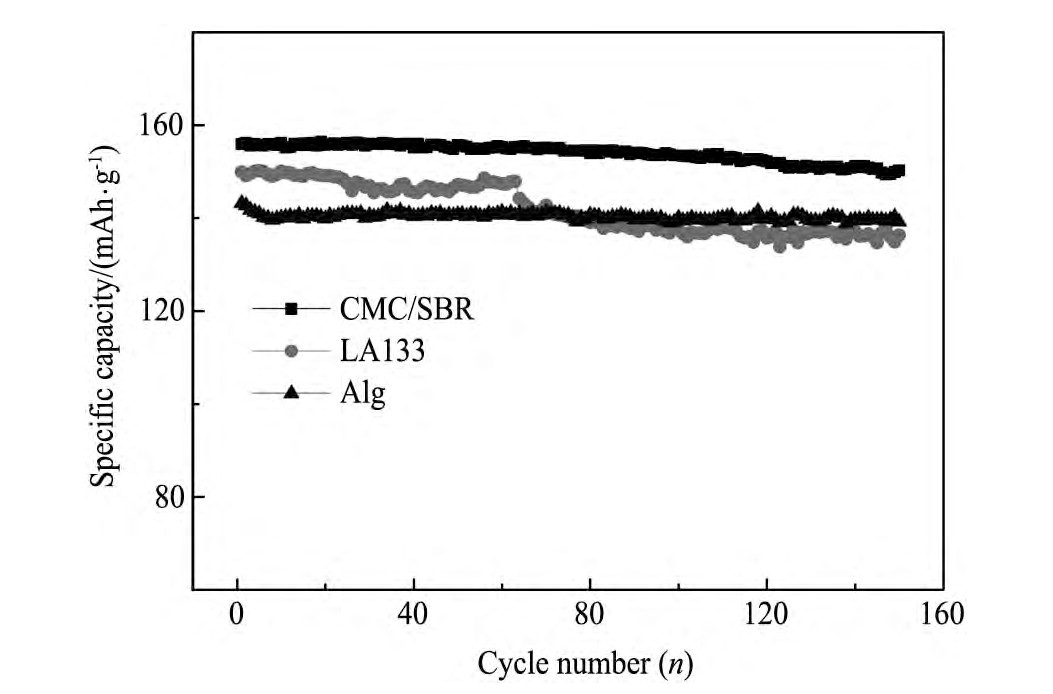
不同水性粘结剂制备的Li Fe PO4电极的循环性能见图5。对比图5中不同Li Fe PO4电极的循环曲线可以发现,CMC/SBR复合粘结剂制备的电极性能最好,在85 m A·g-1(0.5C)的电流密度下可发挥出156 m Ah·g-1的比容量,循环150周后放电比容量仍高达~150.3m Ah·g-1,容量保持率为97%。以LA133为粘结剂制备的Li Fe PO4电极在85 m A·g-1(0.5C)的电流密度下初始放电容量为149.9 m Ah·g-1,但由于实验所用LA133电化学稳定性略差,且以LA133为粘结剂制备的极片裂纹比较明显,随循环次数增加该电极容量下降较快,150周后容量保持率为90.9%;而以Alg为粘结剂制备的Li Fe PO4电极虽容量保持率较高,150周容量保持率为97%,但该电极严重影响了Li Fe PO4电极材料的容量发挥,初始放电比容量仅有~143 m Ah·g-1。
图5 不同水性粘结剂制备的Li Fe PO4电极在0.5C电流密度下的循环稳定性Fig.5 Cycle stability of Li Fe PO4electrodes prepared with different water-based binders at a current density of 0.5C
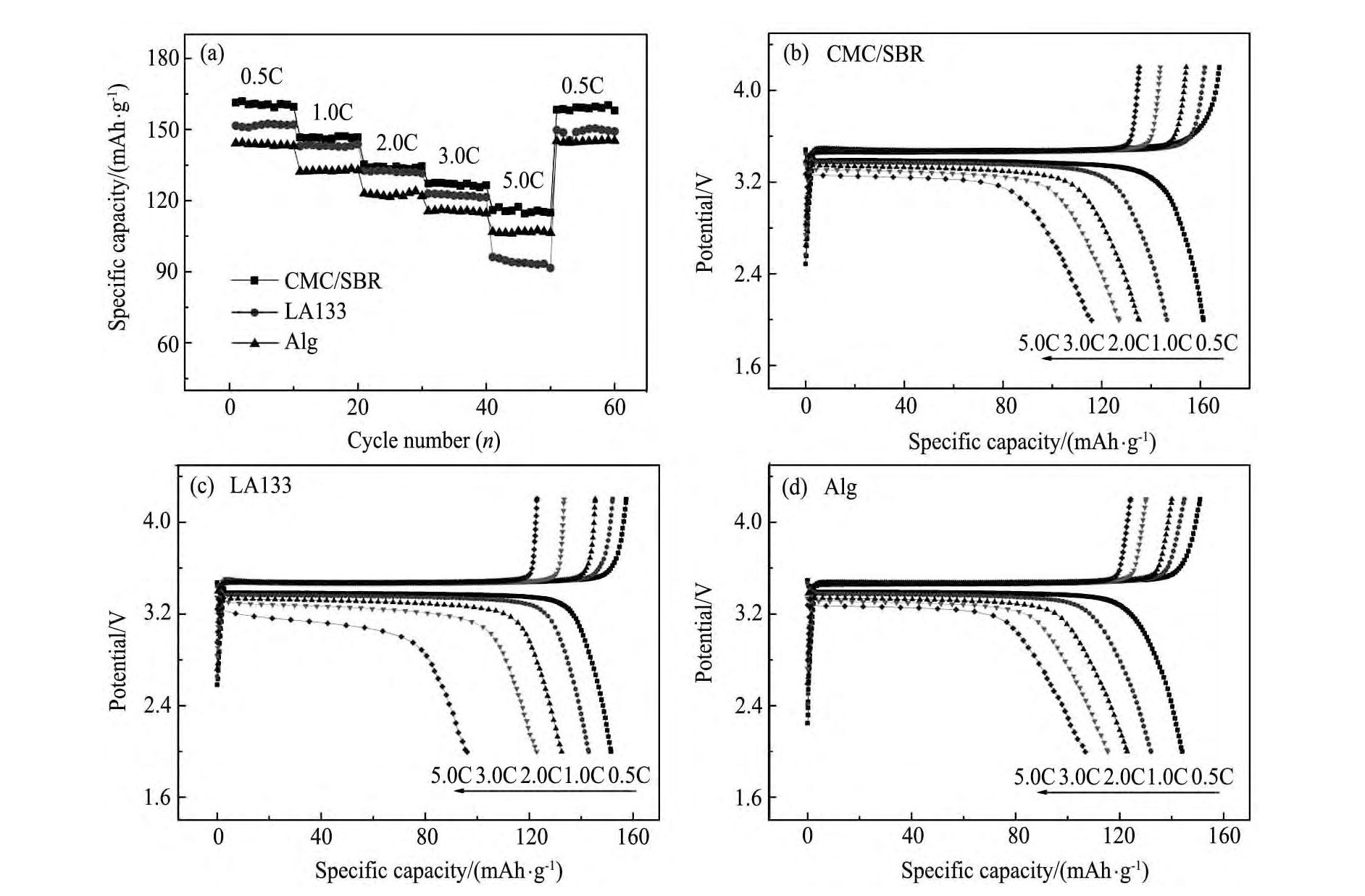
2.3.3 倍率性能
不同水性粘结剂制备的Li Fe PO4电极的倍率性能见图6。从图6可知,经过60次不同电流密度的循环,由不同粘结剂制备的Li Fe PO4电极的放电比容量均可恢复到初始值,显示出良好的倍率循环稳定性;其中CMC/SBR复合粘结剂制备的Li Fe PO4电极在不同倍率下的放电比容量均明显高于其他两类粘结剂制备的电极。当电流密度高达850 m A·g-1(5C)时,以CMC/SBR为粘结剂制备的Li Fe PO4电极仍可发挥出~117m Ah·g-1的放电比容量,而以Alg为粘结剂制备的Li Fe PO4电极放电比容量为106 m Ah·g-1,以LA133为粘结剂制备的Li Fe PO4电极5C放电比容量仅有94 m Ah·g-1。
2.4 循环后结构分析
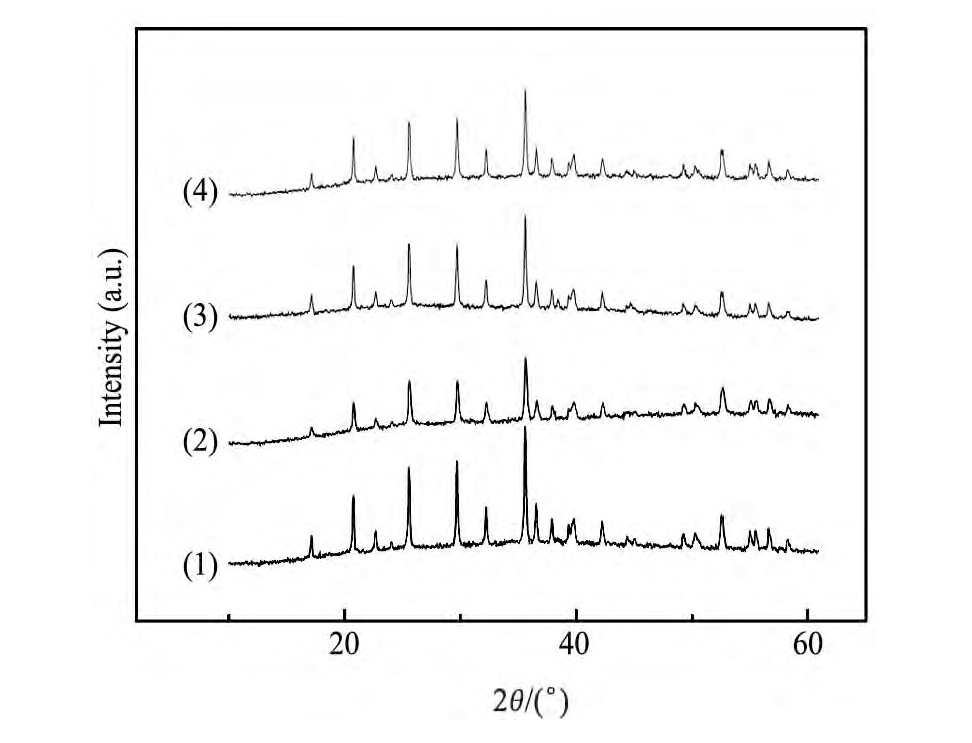
另外,为考察循环过程中水性粘结剂对电极材料结构的影响,对比了原始电极材料和循环150周后的Li Fe PO4电极的XRD图谱,结果如图7所示。对比图7的XRD图谱可以发现,循环150周后,虽然不同粘结剂制备的电极的衍射峰强度略有差异,但衍射峰的位置与循环前电极材料的谱图基本一致,几乎没有检测到其他杂相峰,即循环充放电后电极材料仍保持单一的正交晶系橄榄石型晶体结构,表明本文中以水性粘结剂制备的Li Fe PO4电极在循环过程中,活性物质的结构基本没有受到影响。
图6 不同水性粘结剂制备的Li Fe PO4电极的倍率性能Fig.6 Rate performance of Li Fe PO4electrodes prepared with different water-based binders
图7 不同水性粘结剂制备的Li Fe PO4电极循环前后的XRD图谱Fig.7 XRD patterns of Li Fe PO4electrodes prepared with dif-ferent water-based binders after 150 cycles
(1)Original Li Fe PO4material;(2)CMC/SBR;(3)LA133;(4)Alg
3 结论
考察了常见商业水性粘结剂CMC,SBR,LA133,Alg在自主研发的磷酸铁锂电极材料中的应用性能。实验结果显示粘结剂溶液的粘度、粘结剂的柔韧性及在锂离子电池中的电化学稳定性等均会对极片的电化学性能产生很大影响;且在循环过程中,实验所用水性粘结剂均不会对电极材料的结构产生不良影响。对比制得极片的电化学性能可以发现,由CMC/SBR混合型粘结剂制备的电极综合性能最好,在85 m A·g-1(0.5C)的电流密度下循环150周后,电极的容量保持率为97%;当放电电流密度高达850 m A·g-1(5.0C)时,电极仍可发挥出~115 m Ah·g-1的比容量,表现出很好的应用性能。
参考文献