
J. Cent. South Univ. (2018) 25: 709-718
DOI: https://doi.org/10.1007/s11771-018-3775-y
Absorption performance of DMSA modified Fe3O4@SiO2core/shell magnetic nanocomposite for Pb2+ removal
TIAN Qing-hua(田庆华), WANG Xiao-yang(王晓阳), MAO Fang-fang(毛芳芳), GUO Xue-yi(郭学益)
School of Metallurgy and Environment, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
The purpose of this study is to explore the adsorption performance of meso-2, 3-dimercaptosuccinic acid (DMSA) modified Fe3O4@SiO2 magnetic nanocomposite (Fe3O4@SiO2@DMSA) for Pb2+ ions removal from aqueous solutions. The effects of solution pH, initial concentration of Pb2+ions, contact time, and temperature on the amount of Pb2+ adsorbed were investigated. Adsorption isotherms, adsorption kinetics, and thermodynamic analysis were also studied. The results showed that the maximum adsorption capacity of the Fe3O4@SiO2@DMSA composite is 50.5 mg/g at 298 K, which is higher than that of Fe3O4 and Fe3O4@SiO2 magnetic nanoparticles. The adsorption process agreed well with Langmuir adsorption isotherm models and pseudo second-order kinetics. The thermodynamic analysis revealed that the adsorption was spontaneous, endothermic and energetically driven in nature.
Key words:
lead removal; adsorption; Fe3O4@SiO2 core/shell structure; DMSA modification; magnetic nanocomposite;
Cite this article as:
TIAN Qing-hua, WANG Xiao-yang, MAO Fang-fang, GUO Xue-yi. Absorption performance of DMSA modified Fe3O4@SiO2 core/shell magnetic nanocomposite for Pb2+ removal [J]. Journal of Central South University, 2018, 25(4): 709–718.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3775-y1 Introduction
Heavy metals are generally considered to be a threat toward humans and ecosystems owing to their high potential toxicity [1]. Lead is a strong poison and toxic to vital target organs and body systems, which could cause a wide range of health problems [2, 3]. A number of techniques have been developed to remove the metal ions from wastewater such as chemical precipitation and ion exchange process [4], biotechnology [5], membrane and reverse osmosis processes [6], electrochemical methods [7], and adsorption [8–10]. Among them, adsorption is the best available method due to the convenient operation process, wide applicability, high efficiency, low energy requirement and cost effectiveness [11, 12]. Recently, many research groups have explored several nanoparticles for heavy metal removal, such as alumina [13], carbon [14], silica [15] nanomaterials, because of the ease of their surface functionality modification and their high surface area-to-volume ratio for increased adsorption capacity and efficiency [16].
In the last decade, iron oxide magnetic nanoparticles (MNPs) have been regarded as attractive adsorbents due to their high surface area and the unique advantage of easy separation under external magnetic fields [17]. However, MNPs are unstable and usually tend to aggregate and, therefore, greatly weaken their efficiency for environmental application [18]. It is shown that coating of MNPs with functional groups can prevent the magnetite core from particle aggregation and improve the dispersion stability in suspension medium [19]. Many materials such as chitosan [20], carbon [21], polyrhodanine [22], are considered to be good alternatives for the surface modification of iron oxide MNPs. Among these, SiO2 is deemed to an ideal shell composite because it is stable under acidic conditions and inert to redox reactions [23]. Additionally, the availability of —OH moieties can provide the binding sites for other organic molecules which may further extend heavy metal removal applications [24].
Nowadays, in order to achieve the most effectiveness in adsorption systems, Fe3O4@SiO2 nanoparticles have been functionalized with different materials such as amino, porphyrin, and acetoacetanilide for heavy metal removal from water [25–27]. The dimercaptosuccinic acid (DMSA, C4S2O4H6) has attracted considerable attention as a new class of highly dispersible biocompatible material for Fe3O4 nanoparticles functionalization recently [28, 29]. Besides, the DMSA has an abundance of surface sulfhydryl groups, which can enhance the adsorption capability of magnetic nanoparticles.
Considering the excellent dispersed property and adsorption ability of DMSA, here we explored the DMSA modified Fe3O4@SiO2 core/shell nanoparticles for Pb2+ removal from water by a simple and effective synthesis method. In addition, we investigated the adsorption capacity of Fe3O4@SiO2@DMSA composites for Pb2+ removal with different solution pH, contact time and metal ion concentration. We also studied the adsorption isotherms, kinetics and thermodynamics to understand the mechanism of the lead ions adsorption of as-prepared MNPs adsorbent and explored the effect of MNPs reuse. The comparative analysis of adsorption capacity of naked Fe3O4 MNPs, SiO2 coated Fe3O4 MNPs (Fe3O4@SiO2), and Fe3O4@SiO2@DMSA MNPs were also carried out in the study.
2 Experimental
2.1 Materials
Dimethyl sulfoxide (DMSO), aqueous ammonia (25%–28%), tetraethyl orthosilicate (TEOS), meso-2, 3-dimercaptosuccinic acid (DMSA), ethanol, lead nitrate, sodium hydroxide, 5, 5-Dithiobis-(2-nitrobenzoic acid) (DTNB) were purchased from Aladdin Industrial Corporation (Shanghai, China). Toluene was purchased from Aladdin Industrial Corporation (Shanghai, China). Nitric acid was provided by Xingkong Industrial Corporation (Zhuzhou, China) and hydrochloric acid was provided by Kaixin Industrial Corporation (Hengyang, China). Water used in all experiments was purified using a Milli-Q Plus 185 water purification system (Millipore, Bedford, MA). All chemicals were of analytical grade.
2.2 Synthesis of Fe3O4@SiO2@DMSA core/shell nanoparticles
The Fe3O4 magnetic nanoparticles were supplied by Research Institute for Resource Recycling, (Changsha, China). The detailed description of the synthetic methods of Fe3O4@SiO2 and DMSA modified Fe3O4@SiO2 MNPs were presented in the previous research [30]. To synthesize the Fe3O4@SiO2 nanocomposite,100 mg as-prepared hydrophilic Fe3O4 MNPs were suspended in ethanol (150 mL) under sonication for 40 min. Aqueous ammonia (3 mL) and deionized water (47 mL) were added in sequence to the suspension, and the mixture (with the volume ratio of ethanol-water-ammonia 75.0%-23.5%-1.5%) was sonicated for 40 min. Then, 0.6 mL TEOS was added slowly under mild continuous stirring at room temperature for another 8 h. The silica-coated nanoparticles were isolated by magnetic separation and were washed with ethanol several times. The final product was collected and dried at 60 °C under vacuum.
The DMSA modified Fe3O4@SiO2 nanocomposites were obtained by the following process. 100 mg Fe3O4@SiO2 nanoparticles were dispersed in 10 mL toluene under sonication for 30 min, and then 10 mL DMSO and 250 mg DMSA were added in the solution. The mixture was stirred for 12 h. The final products were collected by magnetic separation, washed with ethanol and deionized water several times, and dried at 60 °C under vacuum.
2.3 Adsorption experiments
Adsorption of Pb2+ ions from aqueous solution was determined in batch experiments. We investigated the effect of pH (2.0–6.0), kinetics time (10–130 min), adsorption isotherms (initial concentration 0–500 mg/L) of Pb2+ ions, and thermodynamics (298–323 K). Analyzing adsorption behavior of the MNPs involved adding 20 mg (Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2@ DMSA) to 20 mL of solution of the Pb2+ ions concentrations at 100 mg/L at 298 K. The pH was maintained at a constant value during adsorption. The contact time was 4 h. The adsorbents were separated by powerful magnets and centrifuging at 5000 r/min for 10 min. The Pb2+ ion concentration adsorbed per gram of adsorbent qt at time t was calculated as follows [31]:
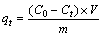 (1)
(1)
where C0 and Ct are the concentrations of the Pb2+ in the solution before and after adsorption, respectively (mg/L); V is the volume of the solution (L), and m is the amount of dry MNPs used (g).
2.4 Desorption experiments
To evaluate the ability to reuse the Fe3O4@ SiO2@DMSA nanoparticles, we investigated the effects of the concentrations of hydrochloric acid (0.05–1.60 mol/L) on the stability of the adsorbents and the amount of desorbed Pb2+ ions. The desorption experiments involved adding 40 mg Pb2+ ion-loaded adsorbents to 20 mL aqueous solution with 0.10 mol/L hydrochloric acid. The mixture was shaken for 3 h to reach desorption equilibrium. Adsorption-desorption cycles were repeated five times by using the same MNPs.
3 Results and discussion
3.1 Characterization of prepared MNPs
In the previous studies, the detailed characterizations of the morphology, structure, functional groups, surface charge, magnetic susceptibility of the Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2@DMSA MNPs were carried out by transmission electron microscopy (TECNAI G2 60-300, FEI), X-ray diffraction (S-3000N, Hitachi), Fourier transform infrared spectrometer(6700, NICOLET), X-ray photoelectron spectroscopy (ESCALAB 250Xi, Thermo Fisher), zeta potential analysis (Nano-2S&MPT-2, Malvern), dynamic laser scattering (Nano-2S&MPT-2, Malvern) and vibrating sample magnetometer (HH-15) [30]. The results revealed that: 1) The size of the DMSA modified Fe3O4@SiO2 MNPs could be tuned with core diameter of 150–200 nm and layer thickness of 15–19 nm. 2) The FTIR and XPS results showed that DMSA molecules were bound to the particle surface via COO— groups. 3) The content of —SH on the surface of the Fe3O4@SiO2@DMSA nanoparticles was calculated to be 0.089 mmol/g. 4) The Fe3O4@SiO2@DMSA nanoparticles exhibited fine colloidal stability, enhanced dispersibility, and optimal magnetization after DMSA modification, which is confirmed by ZETA, DLS, and VSM measurements. The Pb (II) ions were supposed to interact with the adsorbents through the active sites on the surface of MNPs (Scheme 1).
3.2 Results of batch experiments
3.2.1 Effect of pH
The initial pH is an important variable for adsorption of the metal ions [19]. The effect of pH on the adsorption for Pb2+ has been studied in the pH range 2.0–6.0 and is illustrated in Figure 1. The removal efficiency increased with pH 2.0–4.5, which might be ascribed to the competition of Pb2+and Pb(OH)+ ions with H+ ions for the active sites on the MNPs surface [31]. As the alkalinity of solution increases, the —SH and —OH turn into —S— and —O— anions and the adsorption increases gradually until pH>pHzpc (pH at zero point charge). Thereafter, the —SH and —OH completely turn into —S— and —O— anions, with almost no change in adsorption [32]. Considering that Pb2+ will precipitate as hydroxide when the pH up to a certain value, we chose pH=5.5 for further experiments [27].

Scheme 1 Presentation of possible mechanism of prepared MNPs for Pb (II) ions adsorption

Figure 1 Effect of pH on adsorption of Pb2+ ions; initial Pb2+concentration: 100 mg/L, adsorbent dose: 1 mg/mL, reaction temperature: 298 K, contact time: 4 h
3.2.2 Adsorption isotherm
Figure 2 shows the adsorption isotherms of Pb2+ on Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2@ DMSA MNPs at 298 K. The equilibrium capacities of the Pb2+ ions are increased with increasing concentrations until reaching equilibrium. Besides, the maximum capacity of Pb2+ on the MNPs decreases in the order Fe3O4@SiO2@DMSA> Fe3O4@SiO2>Fe3O4, indicating the adsorption capacity is improved after DMSA modification. The observed differences in the maximum capacities of Pb2+ ions are most probably due to the different affinity to interact with —SH and —OH groups of the MNPs [33].
Equilibrium data can be analyzed by widely used adsorption isotherms, Langmuir isotherm and Freundlich isotherm, which provide the basis for the design of adsorption systems. The former is based on the monolayer and homogenized coverage of the adsorbent surface, where all sorption sites are identical and energetically equivalent [34] (Eq. (2)). The Freundlich model assumes multilayer, reversible and non-ideal adsorption at the active sites with exponential distribution of energies, which endorses the heterogeneity of the surface [35] (Eq.(3)).
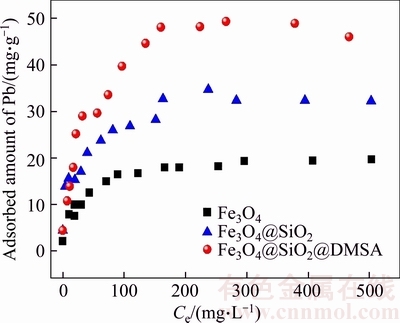
Figure 2 Effect of initial Pb2+concentration on adsorption of Pb2+ ions (pH=5.5, adsorbent dose:1 mg/mL, reaction temperature: 298 K, contact time: 4 h)
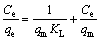 (2)
(2)
 (3)
(3)
where Ce and qe are the concentration and adsorption capacity at equilibrium, and qm and KL are Langmuir constants, representing the maximum adsorption capacity and energy of adsorption (L/mg), respectively. KF and n are Freundlich constants related to adsorption capacity and intensity, respectively.
For each isotherm, the values of qm and KL were calculated from experimental data through Figure 2. The values of KF and n are obtained similarly. The results are presented in Table 1 with the correlation coefficients (R2). It can be seen from Table 1 that the Langmuir model yields a better fit than the Freundlich model by comparing the results of correlation coefficient values. Further, it shows a clear consistency between the calculated (qm) and experimental values (qma) of the adsorption capacity at equilibrium. Therefore, it can be considered that monolayer adsorption takes place at specific sites present on the surface of MNPs, which are energetically identical. It further confirms that chemisorption occurs due to the complexation between Pb2+ ions and the organic groups present on the surface of the nanoadsorbent [36, 37].
Table 1 Parameters of Langmuir and Freundlich adsorption isotherm models for Pb2+ adsorption

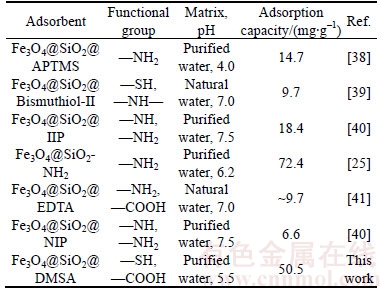
Nowadays, because Fe3O4@SiO2 nanoparticles have been functionalized with different materials for Pb2+ removal, a comparison of the adsorption performance of functionalized Fe3O4@SiO2 nanoparticles are presented in Table 2 [25, 38–41]. Although the comparison is not precise due to the different experimental conditions and the uncompleted data, the conclusion can be acquired that the DMSA modification is basically achieved and has enhanced efficiency for Pb2+ ions adsorption.
Table 2 Applications of functionalized Fe3O4@SiO2 MNPs in Pb2+ removal
3.2.3 Adsorption kinetics
Figure 3 shows the effects of contact time on the adsorption of the Pb2+ ions. It is found that the Pb2+ adsorption of Fe3O4@SiO2@DMSA MNPs is very fast, and the process almost completely reaches equilibrium within 30 min, which is shorter than that of Fe3O4@SiO2, and Fe3O4. The high adsorption rate can be attributed to the presence of sufficient adsorption sites and ease of their accessibility as well as strong complexation ability towards Pb2+ ions [38].

Figure 3 Effect of time on adsorption of Pb2+ ions (pH=5.5, initial Pb2+concentration: 100 mg/L, adsorbent dose: 1 mg/mL, reaction temperature: 298 K)
The adsorption kinetics of metal ions with MNPs was investigated by Lagergren pseudo-first- order (Eq.(4)) and pseudo-second-order (Eq.(5)) models [42]:
 (4)
(4)
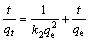 (5)
(5)
where k1 (min–1) and k2 (g mg–1 min–1) are the kinetic rate constants for the pseudo-first-order and the pseudo-second-order models. Then qe and qt are the amounts of Pb2+ adsorbed at equilibrium and at time t, respectively.
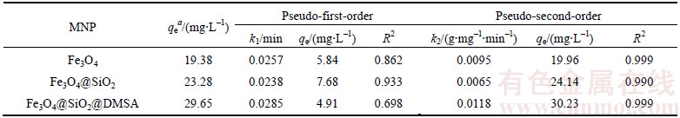
The kinetic adsorption data are fitted to Eqs.(3) and (4), and the calculated results are depicted in Table 3. As it is clear from Table 3, the correction coefficients (R2) for the second order kinetic model are greater than 0.99, suggesting the applicability of this kinetic equation and confirms the second order nature of the adsorption phenomenon of Pb2+ ions on the MNPs. The second-order nature of the adsorption processes confirms that the Pb2+ ions have been adsorbed via chemical reactions [43]. This observation is consistent with this fact that functional groups on the surface of the MNPs can adsorb the Pb2+ ions via complexation reaction.
Table 3 Parameters of applied kinetic models for Pb2+ adsorption
3.2.4 Adsorption thermodynamics
Entropy and energy factors should be considered in order to determine what processes will occur spontaneously in engineering practice. Figure 4 shows the effects of temperature on the adsorption of the Pb2+ ions by MNPs. The improved sorption performance of MNPs with elevated temperature elucidates that the adsorption process is thermodynamically driven.

Figure 4 Effect of temperature on adsorption of Pb2+ ions (Initial Pb2+concentration:100 mg/L, pH=5.5, adsorbent dose: 1 mg/mL, contact time: 4 h)
The Gibbs free energy indicates the degree of spontaneity of the adsorption process and higher negative value reflects a more energetically favorable adsorption. The Gibbs free energy change of adsorption is defined as
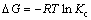 (6)
(6)
where R is the universal gas constant (8.314 J/(mol·K)) and T is the absolute temperature in Kelvin [44]. The equilibrium constant may be expressed in terms of standard enthalpy change of adsorption (△H) and entropy change of adsorption (△S) as a function of temperature. The relationship between the KL and temperature is given by the Vant Hoff equation:
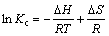 (7)
(7)
△H and △S can be obtained from the slope and intercept of the plot of ln Kc versus 1/T [45] (Figure 5). Thermodynamic parameters quantified with the measured data under a preset range of temperatures are listed in Table 4. △G at all temperatures is a negative value, confirming the spontaneous nature and the thermodynamically favorable adsorption. The positive value of △H implies that the interaction between Pb2+ ions and MNPs is endothermic in nature, while the positive value of △S may be attributed to the increase in randomness at the solid-liquid interface [46]. This increase results from the extra translational entropy gained by the water molecules previously adsorbed onto MNPs but displaced by Pb2+ions. It is noteworthy that adsorption process with △G values between –80 and –400 kJ/mol corresponds to chemisorptions [47, 48]. From the △G values obtained in this study, it can be deduced that the adsorption mechanism is dominated by physisorption [47]. Thus, the adsorption process cannot be simply attributed to a single chemisorption or physisorption. The physisorption may be contributed to the MNPs’ high surface area-to-volume ratio for Pb2+ adsorption.
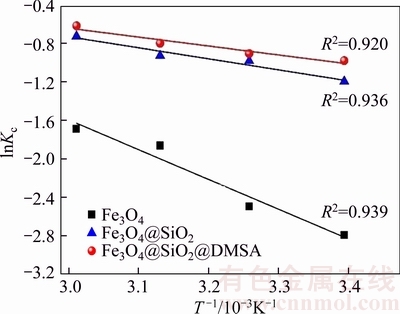
Figure 5 lnKc vs 1/T plots
Table 4 Estimated values of thermodynamic parameters at different temperatures

3.2.5 Desorption and repeated use
The different concentrations of H+ in solution to determine the best conditions for reusing MNPs were investigated (Figure 6). The adsorbents are corroded by the H+, which can be seen from the contents of the released Fe3+ ions. After SiO2 coating, the contents of the released Fe3+ ions are reduced dramatically, indicating that the SiO2 shell can prevent the MNPs corrosion by H+ efficiently. Considering the desorption efficiencies which are more than 90% with 0.1 mol/L H+ and the corrosion effect of the MNPs, the concentration of 0.1 mol/L H+ was selected in the desorption process. The Pb2+ ions adsorption capacities of MNPs decrease at first recycle but change less after second cycle, which indicates the reusability of the MNPs adsorbents (Figure 7). Our recyclability studies suggest that nano-adsorbent can be repeatedly used as an efficient adsorbent in water treatment.

Figure 6 Contents of released Fe3+ ions at different H+ concentrations of desorption
4 Conclusions
The DMSA modified Fe3O4@SiO2 nanocomposites were developed for the removal of Pb2+from aqueous solutions in this study. This results show that DMSA modified Fe3O4@SiO2 nanocomposites could be used for the removal of Pb2+ ions from water in a very short time with high removal efficiency.

Figure 7 Effect of recycle numbers on adsorption of Pb2+ ions (pH=5.5, adsorbent dose: 1 mg/mL,initial Pb2+ concentration: 100mg/L, reaction temperature: 298 K, contact time: 4 h)
1) The maximum enrichment capacity of Fe3O4@SiO2@DMSA nanoparticles was 50.5 mg/g at 298 K, pH=5.5, which is higher than that of Fe3O4 and Fe3O4@SiO2 magnetic nanoparticles.
2) Adsorption was very rapid and equilibrium was achieved in less than 30 min, and the correction coefficients (R2) for the pseudo-second-order kinetic model was greater than 0.99.
3) The Langmuir and Freundlich adsorption models were used to express the sorption phenomenon, with a better fitting to the Langmuir model than the Freundlich model.
4) The thermodynamics of Pb2+ adsorption onto the MNPs confirmed the endothermic nature of the adsorption process, the spontaneity and energetically driven in nature.
5) Desorption and regeneration studies indicated that the SiO2 shell could prevent the MNPs corrosion by H+ efficiently, and nanoadsorbents could be used repeatedly.
References
[1] BRZOSTOWSKI A, FALANDYSZ J, JARZY SKA G, ZHANG D. Bioconcentration potential of metallic elements by Poison Pax (Paxillus involutus) mushroom [J]. Journal of Environmental Science and Health Part A, 2011, 46(4): 378–393.DOI: 10.1080/10934529.2011.542387.
SKA G, ZHANG D. Bioconcentration potential of metallic elements by Poison Pax (Paxillus involutus) mushroom [J]. Journal of Environmental Science and Health Part A, 2011, 46(4): 378–393.DOI: 10.1080/10934529.2011.542387.
[2] WANG Xin-mei, LIU Wen-jun, ZHANG Rong, ZHOU Yi-kai. Effects of exposure to low-level lead on spatial learning and memory and the expression of mGluR1, NMDA receptor in different developmental stages of rats [J]. Toxicology and Industrial Health, 2013, 29(8): 686–696. DOI: 10.1177/0748233712436641.
[3] PATRA R C, RAUTRAY A K, SWARUP D. Oxidative stress in lead and cadmium toxicity and its amelioration [EB/OL]. [2011-04-20].http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3087445/.
[4] FU Feng-lian, WANG Qi. Removal of heavy metal ions from wastewaters: A review [J]. Journal of Environmental Management, 2011, 92(3): 407–418. DOI: 10.1016/ j.jenvman.2010.11.011.
[5] HENG Yan, YANG Chun-ping, HE Hui-jun, ZENG Guang-ming, ZHAO Kun, YAN Zhou. Biosorption of Pb (II) ions from aqueous solutions by waste biomass from biotrickling filters: Kinetics, isotherms, and thermodynamics [J]. Journal of Environmental Engineering, 2016, 142(9): C4015001. DOI: 10.1061/(ASCE)EE.1943-7870.0000956.
[6] KANGMIN C,SEUNG J K,JIHEE M, JAEWEON C. Combined coagulation-disk filtration process as a pretreatment of ultrafiltration and reverse osmosis membrane for wastewater reclamation: An autopsy study of a pilot plant [J]. Water Research, 2012, 46(6): 1803–1816. DOI: 10.1016/ j.watres.2011.12.062.
[7] LIU Yao-xing, YAN Jun-mei, YUAN Dong-xing, LI Quan-long,WU Xiao-yun. The study of lead removal from aqueous solution using an electrochemical method with a stainless steel net electrode coated with single wall carbon nanotubes [J]. Chemical Engineering Journal, 2013, 218: 81–88. DOI: 10.1016/j.cej.2012.12.020.
[8] NAJAFI M, YOUSEFI Y, RAFATI A A. Synthesis, characterization and adsorption studies of several heavy metal ions on amino-functionalized silica nano hollow sphere and silica gel [J]. Separation and Purification Technology, 2012, 85: 193–205. DOI: 10.1016/j.seppur.2011. 10.011.
[9] TIAN Qing-hua, GUO Xue-yi. Electroless copper plating on microcellular polyurethane foam [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(s1): 283–287. DOI: 10.1016/S1003-6326(10)60057-X.
[10] TIAN Qing-hua, GUO Xue-yi. Manufacturing microporous foam zinc materials with high porosity by electrodeposition [J]. Journal Wuhan University of Technology, Materials Science Edition, 2011, 26(5): 843–846. DOI: 10.1007/ s11595-011-0322-1.
[11] BARAKAT M A. New trends in removing heavy metals from industrial wastewater [J]. Arabian Journal of Chemistry, 2011, 4(4): 361–377. DOI: 10.1016/j.arabjc.2010.07.019.
[12] MOHAN D, SARSWAT A, OK Y S, CHARLES U, PITTMAN J. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent–A critical review [J]. Bioresource Technology, 2014, 160: 191–202. DOI: 10.1016/j.biortech. 2014.01.120.
[13] ABBAS A, MOHAMMAD S T, HASAN B. Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2, 4-dinitrophenylhydrazine [J]. Journal of Hazardous Materials, 2010, 181(1–3): 836–844. DOI: 10.1016/j.jhazmat.2010.05.089.
[14] KRYSTYNA P,  B. Comparative study of heavy metal ions sorption onto activated carbon, carbon nanotubes, and carbon-encapsulated magnetic nanoparticles [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 362(1–3): 102–109. DOI: 10.1016/j.colsurfa.2010.03. 047.
B. Comparative study of heavy metal ions sorption onto activated carbon, carbon nanotubes, and carbon-encapsulated magnetic nanoparticles [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 362(1–3): 102–109. DOI: 10.1016/j.colsurfa.2010.03. 047.
[15] SAYARI A, HAMOUDI S, YANG Yong. Applications of pore-expanded mesoporous silica. 1. Removal of heavy metal cations and organic pollutants from wastewater [J]. Chemical Materials, 2005, 17(1): 212–216. DOI: 10.1021/ cm048393e.
[16] HUA Ming, ZHANG Shu-juan, PAN Bing-cai, ZHANG Wei-ming, LV Lu, ZHANG Quan-xing. Heavy metal removal from water/wastewater by nanosized metal oxides: A review [J]. Journal of Hazardous Materials, 2012, 211–212: 317–331. DOI: 10.1016/j.jhazmat.2011.10.016.
[17] WANG Li-xia, LI Jian-chen, JIANG Qing, ZHAO Li-jun. Water-soluble Fe3O4 nanoparticles with high solubility for removal of heavy-metal ions from waste water [J]. Dalton Transactions, 2012, 41: 4544–4551. DOI: 10.1039/ c2dt11827k.
[18] HU Jing, CHEN Guo-hua, IRENE M C. Selective removal of heavy metals from industrial wastewater using maghemite nanoparticle: Performance and mechanisms [J]. Journal of Environmental Engineering, 2006, 132(7): 709–715. DOI: 10.1061/(ASCE)0733-9372(2006)132:7(709).
[19] GE Fei, LI Meng-meng, YE Hui, ZHAO Bao-xiang. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles [J]. Journal of Hazardous Materials, 2012, 211–212: 366–372. DOI: 10.1016/j.jhazmat.2011.12.013.
[20] LIU Xiao-wang, HU Qi-yan, FANG Zhen, ZHANG Xiao-jun, ZHANG Bei-bei. Magnetic chitosan nanocomposites: A useful recyclable tool for heavy metal ion removal [J]. Langmuir, 2009, 25(1): 3–8. DOI: 10.1021/la802754t.
[21] BYSTRZEJEWSKI M, PYRZY SKA K, HUCZKO A, LANGE H. Carbon-encapsulated magnetic nanoparticles as separable and mobile sorbents of heavy metal ions from aqueous solutions [J]. Carbon, 2009, 47(4): 1201–1204. DOI: 10.1016/j.carbon.2009.01.007.
SKA K, HUCZKO A, LANGE H. Carbon-encapsulated magnetic nanoparticles as separable and mobile sorbents of heavy metal ions from aqueous solutions [J]. Carbon, 2009, 47(4): 1201–1204. DOI: 10.1016/j.carbon.2009.01.007.
[22] JOOYOUNG S, HYEYOUNG K, JYONGSIK J. Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles [J]. Journal of Colloid and Interface Science, 2011, 359(2): 505–511. DOI: 10.1016/j.jcis.2011.04.034.
[23] ASHTARI P, HE Xiao-xiao, WANG Ke-min, GONG Ping. An efficient method for recovery of target ssDNA based on amino-modified silica-coated magnetic nanoparticles [J]. Talanta, 2005, 67(3): 548–554. DOI: 10.1016/j.talanta.2005. 06.043.
[24] YUAN Qing, LI Nan, CHI Yue, GENG Wang-chang, YAN Wen-fu, ZHAO Ying, LI Xiao-tian, DONG Bin. Effect of large pore size of multifunctional mesoporous microsphere on removal of heavy metal ions [J]. Journal of Hazardous Materials, 2013, 254–255: 157–165. DOI: 10.1016/j.jhazmat. 2013.03.035.
[25] WANG Jia-hong, ZHENG Shou-rong, SHAO Yun, LIU Jing-liang, XU Zhao-yi, ZHU Dong-qiang. Amino- functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal [J]. Journal of Colloid and Interface Science, 2010, 349(1): 293–299. DOI: 10.1016/j.jcis.2010.05.010.
[26] SUN Lei, LI Yao-xian, SUN Ming-da, WANG Heng-guo, XU Shu-fei, ZHANG Chao-qun, YANG Qing-biao. Porphyrin-functionalized Fe3O4@SiO2 core/shell magnetic colorimetric material for detection, adsorption and removal of Hg2+ in aqueous solution [J]. New Journal of Chemistry, 2011, 35: 2697–2704. DOI: 10.1039/C1NJ20307J.
[27] SHARMA R K, PURI A, MONGA Y, ADHOLEYA A. Acetoacetanilide-functionalized Fe3O4 nanoparticles for selective and cyclic removal of Pb2+ ions from different charged wastewaters [J]. Journal of Materials Chemistry A, 2014, 2: 12888–12898. DOI: 10.1039/C4TA01815J.
[28]  S, ZOTES T, L
S, ZOTES T, L ZARO F, MORALES M, BARBER D. Long term biotransformation and toxicity of dimercaptosuccinic acid-coated magnetic nanoparticles support their use in biomedical applications [J]. Journal of Controlled Release, 2013, 171(2): 225–233. DOI: 10.1016/ j.jconrel.2013.07.019.
ZARO F, MORALES M, BARBER D. Long term biotransformation and toxicity of dimercaptosuccinic acid-coated magnetic nanoparticles support their use in biomedical applications [J]. Journal of Controlled Release, 2013, 171(2): 225–233. DOI: 10.1016/ j.jconrel.2013.07.019.
[29] JAISWAL M K, MEHTA S, BANERJEE R, BAHADUR D. A comparative study on thermoresponsive magnetic nanohydrogels: Role of surface-engineered magnetic nanoparticles [J]. Colloid and Polymer Science, 2012, 290(7): 607–617. DOI: 10.1007/s00396-011-2572-z.
[30] GUO Xue-yi, MAO Fang-fang, WANG Wei-jia, YANG Ying, BAI Zhi-ming. Sulfhydryl-modified Fe3O4@SiO2 core/shell nanocomposite: Synthesis and toxicity assessment in vitro [J]. ACS Applied Materials & Interfaces, 2015, 7: 14983–14991. DOI: 10.1021/acsami.5b03873.
[31] SINGH S, BARICK K C, BAHADUR D. Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens [J]. Journal of Hazardous Materials, 2011, 192: 1539–1547. DOI: 10.1016/j.jhazmat. 2011.06.074.
[32] JIN Xin-liang, YU Cui, LI Yan-feng, QI Yong-xin,YANG Liu-qing, ZHAO Guang-hui, HU Huai-yuan. Preparation of novel nano-adsorbent based on organic-inorganic hybrid and their adsorption for heavy metals and organic pollutants presented in water environment [J]. Journal of Hazardous Materials, 2011, 186(2): 1672–1680. DOI: 10.1016/j.jhazmat. 2010.12.057.
[33] ZARGOOSH K, ABEDINI H, ABDOLMALEKI A, MOLAVIAN M. Effective removal of heavy metal ions from industrial wastes using thiosalicylhydrazide-modified magnetic nanoparticles [J]. Industrial & Engineering Chemistry Research, 2013, 52(42): 14944–14954. DOI: 10.1021/ie401971w.
[34] LANGMUIR I. The constitution and fundamental properties of solids and liquids. II. Liquids [J]. Journal of the American Chemical Society, 1917, 39(9): 1848–1906. DOI: 10.1021/ ja02268a002.
[35] HUANG Yao-hui, HSUEH C L, HUANG Chun-ping, SU Liang-chih,CHEN Chuh-yung. Adsorption thermodynamic and kinetic studies of Pb (II) removal from water onto a versatile Al2O3-supported iron oxide [J]. Separation and Purification Technology, 2007, 55(1): 23–29. DOI: 10.1016/ j.seppur.2006.10.023.
[36] KUANG Shao-ping, WANG Zhao-zhan, LIU Jie, WU Zhan-chao. Preparation of triethylene-tetramine grafted magnetic chitosan for adsorption of Pb(II) ion from aqueous solutions [J]. Journal of Hazardous Materials, 2013, 260: 210–219. DOI: 10.1016/j.jhazmat.2013.05.019.
[37] LUO Xu-biao, LIU Ling-ling, DENG Fang, LUO Sheng-lian. Novel ion-imprinted polymer using crown ether as a functional monomer for selective removal of Pb(II) ions in real environmental water samples [J]. Journal of Material Chemistry A, 2013, 1: 8280–8286. DOI: 10.1039/ C3TA11098B.
[38] HAO Yong-mei, CHEN Man, HU Zhong-bo. Effective removal of Cu (II) ions from aqueous solution by amino- functionalized magnetic nanoparticles [J]. Journal of Hazardous Materials, 2010, 184: 392–399. DOI: 10.1016/ j.jhazmat.2010.08.048.
[39] SULEIMAN J S, HU Bin, PENG Han-yong, HUANG Chao-zhang. Separation/preconcentration of trace amounts of Cr, Cu and Pb in environmental samples by magnetic solid- phase extraction with Bismuthiol-II-immobilized magnetic nanoparticles and their determination by ICP-OES [J]. Talanta, 2009, 77(5): 1579–1583. DOI: 10.1016/j.talanta. 2008.09.049.
[40] ZHANG Ming-lei, ZHANG Zhao-hui, LIU Yu-nan, YANG Xiao, LUO Li-juan, CHEN Ju-tao, YAO Shou-zhuo. Preparation of core-shell magnetic ion-imprinted polymer for selective extraction of Pb (II) from environmental samples [J]. Journal of Chemical Engineering, 2011, 178: 443–450. DOI: 10.1016/j.cej.2011.10.035.
[41] SINHA A, JANA N R. Functional, mesoporous, superparamagnetic colloidal sorbents for efficient removal of toxic metals [J]. Chemical Communication, 2012, 48: 9272–9274. DOI: 10.1039/c2cc33893a.
[42] ZHANG Yuan-bo, LI Peng, ZHOU You-lian, HAN Gui-hong, LI Guang-hui, XU Bin, JIANGTao. Adsorption of lignite humic acid onto magnetite particle surface [J]. Journal of Central South University, 2012, 19(7): 1967–1972. DOI: 10.1007/s11771-012-1233-9.
[43] NABI S A, SHAHADAT M, SHALLA A, KHAN A. Removal of heavy metals from synthetic mixture as well as pharmaceutical sample via cation exchange resin modified with Rhodamine B: Its thermodynamic and kinetic studies [J]. Clean-Soil Air Water, 2011, 39(12): 1120–1128. DOI: 10.1002/clen.201000314.
[44] SMITH J, van NESS H, ABBOTT M. Introduction to chemical engineering thermodynamics [M]. Singapore: Mcgraw-Hill, 1987.
[45] AKSU Z. Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel (II) ions onto Chlorella vulgaris [J]. Process Biochemical, 2002, 38: 89–99. DOI: 10.1016/S0032-9592(02)00051-1.
[46] NASHAAT N, NASSAR. Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents [J]. Journal of Hazardous Materials, 2010, 184: 538–546. DOI: 10.1016/j.jhazmat.2010.08.069.
[47] SEKI Y, YURDAKOC K. Adsorption of promethazine hydrochloride with KSF montmorillonite [J]. Adsorption, 2006, 12: 89–100. DOI: 10.1007/s10450-006-0141-4.
[48] YU Ying, ZHUANG Yuan-yi, WANG Zhong-hua. Adsorption of water-soluble dye onto functionalized resin [J]. Journal of colloid and interface science, 2001, 242(2): 288–293. DOI: 10.1006/jcis.2001.7780.
(Edited by HE Yun-bin)
中文导读
DMSA改性Fe3O4@SiO2核壳结构磁性纳米复合材料对Pb2+的吸附行为
摘要:本研究旨在探究2,3-二巯基丁二酸(DMSA)改性的Fe3O4@SiO2核壳结构纳米复合材料(Fe3O4@SiO2@DMSA)对水溶液中Pb2+的去除效果。考察了溶液pH、初始Pb2+浓度、吸附时间、温度对Pb2+吸附量的影响。结果表明,在298 K的条件下Fe3O4@SiO2@DMSA复合材料对Pb2+的饱和吸附量为50.5 mg/g,明显高于Fe3O4和Fe3O4@SiO2两种磁性纳米材料。 Fe3O4@SiO2@DMSA对Pb2+的吸附过程符合Langmuir等温吸附模型,为准二级动力学吸附。热力学分析显示Fe3O4@SiO2@DMSA对Pb2+的吸附为自发的、吸热的过程。
关键词:除铅;吸附;Fe3O4@SiO2核壳结构;DMSA改性;磁性纳米复合材料
Foundation item: Project(2013DFA51290) supported by International S&T Cooperation Program of China
Received date: 2016-07-27; Accepted date: 2016-12-20
Corresponding author: GUO Xue-yi, PhD, Professor; Tel: +86–731–88877863; E-mail: xyguo@csu.edu.cn
Abstract: The purpose of this study is to explore the adsorption performance of meso-2, 3-dimercaptosuccinic acid (DMSA) modified Fe3O4@SiO2 magnetic nanocomposite (Fe3O4@SiO2@DMSA) for Pb2+ ions removal from aqueous solutions. The effects of solution pH, initial concentration of Pb2+ions, contact time, and temperature on the amount of Pb2+ adsorbed were investigated. Adsorption isotherms, adsorption kinetics, and thermodynamic analysis were also studied. The results showed that the maximum adsorption capacity of the Fe3O4@SiO2@DMSA composite is 50.5 mg/g at 298 K, which is higher than that of Fe3O4 and Fe3O4@SiO2 magnetic nanoparticles. The adsorption process agreed well with Langmuir adsorption isotherm models and pseudo second-order kinetics. The thermodynamic analysis revealed that the adsorption was spontaneous, endothermic and energetically driven in nature.


