3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-
5-酮的合成工艺
李谷才1, 2,尹端沚2,练文柳3,汪勇先2
(1. 湖南工程学院 化学化工系,湖南 湘潭,411104;
2. 中国科学院上海应用物理研究所 放射性药物研究中心,上海 嘉定,201800;
3. 中南大学 化学化工学院, 湖南 长沙,410083)
摘 要:
摘 要:以3-甲氧基苯酚、4-酮-3-甲酸甲酯哌啶盐酸盐和对羟基苯甲醛为原料,通过分子间环加成反应和N-烷基化反应,合成了一种潜在的多巴胺D4受体拮抗剂3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮。采用红外光谱、质谱、氢核磁共振谱和元素分析等手段对中间体及产物进行表征。研究结果表明:在分子间环加成反应中,当反应物3-甲氧基苯酚、4-酮-3-甲酸甲酯哌啶盐酸盐与硫酸的物质的量比为1?1?30、反应时间为48 h时,最高收率为49.2%;在N-烷基化反应中,当反应物8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮、4-羟基苯甲醛与三乙酸基硼氢化钠的物质的量比为2?4?5、反应时间为20 h时,最高收率为51.8%。
关键词:
苯并吡喃[3, 4-c]吡啶-5-酮;多巴胺D4受体;合成;
中图分类号:O626 文献标识码:A 文章编号:1672-7207(2007)06-1129-06
Synthesis technique of 3-(4-hydroxybenzyl)-8- methoxy-1, 2, 3, 4- tetrahydrochromeno[3, 4-c]pyridin-5-one
LI Gu-cai1, 2, YIN Duan-zhi2, LIAN Wen-liu3, WANG Yong-xian2
(1. Department of Chemistry and Chemical Engineering, Hunan Institute of Engineering, Xiangtan 411104, China;
2. Research Center of Radiopharmaceuticals, Shanghai Institute of Applied Physics, Chinese Academy of Sciences,
Jiading 201800, China;
3. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: 3-(4-Hydroxybenzyl)-8-methoxy-1, 2, 3, 4-tetrahydrochromeno[3, 4-c]pyridin-5-one was prepared as a potential dopamine D4 receptor antagonist from 3-methoxyphenol, methyl-4-oxo-3-piperidine carboxylate hydrochloride and 4-hydroxybenzaldehyde through a two-step reaction and their structures were confirmed by infrared spectroscopy (IR), electrospray ionisation-mass spectrometry (ESI-MS), 1H nuclear resonance spectroscopy (NMR) and elemental analysis. The results show that 8-methoxy-1, 2, 3, 4-tetrahydrochromeno [3, 4-c]pyridin-5-one(I) is the product of the intermolecular cycloaddition reaction between 3-methoxyphenol and methyl-4-oxo-3-piperidine carboxylate hydrochloride with yield of 49.2% under the conditions as follows: the molar ratio of n(3-methoxyphenol)? n(methyl-4-oxo-3-pioerdine carboxylate hydrochloride)?n(sulfuric acid) is 1?1?30, and reaction time is 48 h; and 3-(4-hydroxybenzyl)-8-methoxy-1, 2, 3, 4-tetrahydrochromeno[3, 4-c]pyridin-5-one is the product of the N-alkylation reaction with yield of 51.8% under the conditions as follows: the molar ratio of n(I)?n(4-hydroxy benzaldehyde)?n(sodium triacetoxyborohydride) is 2?4?5, and reaction time is 20 h.
Key words: chromeno[3, 4-c]pyridin-5-one; dopamine D4 receptor; synthetic
精神分裂症是一种非常复杂的慢性神经疾病,成年人中约有0.7%的人患有该病。多巴胺功能亢进可能是引起精神分离症的主要原因之一。多巴胺作为一种内源性儿茶酚胺类神经递质,它对脑、心血管、肾、肾上腺等重要器官机能具有调节作用[1]。多巴胺主要通过多巴胺受体发挥作用。多巴胺受体分为D1和D2样受体两大类型,D1样受体包括D1受体、D5受体,D2样受体又可分为D2受体、D3受体、D4受体3种亚型。多巴胺D4受体属于G蛋白偶联类的受体,由387个氨基酸组成,其肽链跨越细胞膜7次,肽链的氨基端(N端)在细胞外,羧基端(C端)在细胞内。D4受体与D2受体同属第11染色体,在11P15位置上。D4受体与D2受体、D3受体分别具有41%和39%的同源性。多巴胺D4受体在精神分裂症病因发展中起着重要作用,编码D4受体的基因与精神分裂症之间可能存在重要联系[2-4]。
D4受体基于它的药理学性质和它的分布轮廓,已经揭示出可以作为治疗靶以发展对精神分裂症的治疗。传统的抗精神病药物虽对精神分裂症的阳性和阴性症状都有疗效,但它们会产生锥体外系副作用,而非典型抗精神病药物对精神分裂症有比较好的疗效且不产生或很少产生锥体外系副作用。近年来,研究报道NGD 94-1, U-101387, PB-12, L-745870,RBI- 257,PNU-101958, YM-50001,YM-4361,S18126,NRA0045,FMTP,FHTP和FDTP等多种多巴胺D4受体拮抗剂。苯并吡喃[3, 4-c]吡啶-5-酮类化合物作为一类结构独特的多巴胺受体配基,其部分衍生物对多巴胺D4受体表现出了较高的亲和性和选择性,显示可以作为潜在的抗精神分裂症药物[5-10]。为了寻找对D4受体具有更高亲和性与选择性的配基,根据D4受体与精神分裂症的关系[11-14]及苯并吡喃类化合物结构-活性关系研究[15]和一系列苯并吡喃[3, 4-c]吡啶-5-酮类化合物的合成[16],本文在原来合成路线的基础上,对3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮的合成工艺进行优化。
1 实 验
1.1 主要仪器与试剂
主要仪器为:AVANCE 500核磁共振仪(BRUKER);AVATAR 370 FT-IR红外光谱仪(Thermo Nicolet,KBr压片);MicroMass GCTCA 055质谱仪;WRS-1A 数字熔点仪(上海晶科物理光学仪器有限公司制造),EL III元素分析仪。
主要试剂为:3-甲氧基苯酚,4-酮-3-甲酸甲酯哌啶盐酸盐,三乙酸基硼氢化钠购自Acros 化学试剂公司;对羟基苯甲醛,1,3-二甲基-2-咪唑啉酮,购自Fluka 化学试剂公司;其他试剂,购自上海化学试剂公司。
1.2 合成路线
3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮的合成路线如图1所示。
1.3 实验步骤
1.3.1 8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶- 5-酮(I)的合成
将3-甲氧基苯酚0.30 mL(2.767 mmol)和4-酮-3-
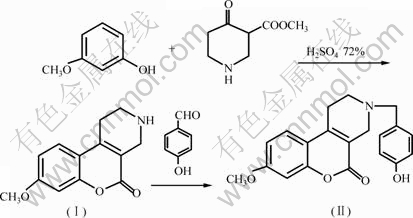
图1 3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮的合成
Fig.1 Synthesis of 3-(4-hydroxybenzyl)-8-methoxy-1, 2, 3, 4- tetrahydrochromeno[3, 4-c] pyridin-5-one
甲酸甲酯哌啶盐酸盐0.535 7 g(2.767 mmol)混合,在冰水浴中冷却至4 ℃后向混合物中滴加72%的硫酸溶液2.0 mL,搅拌,并保持混合物温度低于4 ℃至硫酸滴加完毕。然后,在室温下反应48 h后,向反应体系中加入冰水2.0 mL,用浓氨水调节混合液的pH至8~10。继续搅拌,生成灰白色沉淀。过滤,干燥,得灰色固体。将所得固体研碎,加入稀碱性溶液,搅拌3 h后过滤,用10%的甲醇水溶液洗涤,干燥,用乙腈重结晶,得8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮(Ⅰ) 0.314 5 g,收率为49.2%。
1.3.2 3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮(Ⅱ)的制备
取8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮(Ⅰ)0.110 0 g(0.475 7 mmol)溶于2.0 mL THF中,加入4-羟基苯甲醛51.5 mL(0.475 7 mmol)、冰乙酸(40 mL)、1,3-二甲基-2-咪唑啉酮(1.1 mL),搅拌10 min后加入三乙酸基硼氢化钠0.151 2 g(0.713 5 mmol),在室温下搅拌20 h后减压蒸馏除去溶剂,加入乙腈3 mL,析出沉淀,过滤,洗涤,干燥后重结晶,得3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮(Ⅱ)0.078 2 g,收率为48.7%。
2 反应产物的表征
中间体(Ⅰ)及目标化合物(Ⅱ)的1H-NMR,IR和MS分别如图2和图3所示。
2.1 8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮(Ⅰ)的表征
中间体(Ⅰ)为白色固体,熔点为179~181 ℃;1H NMR (DMSO-d6, 500 MHz)化学位移δ为:2.71 (t, J=5.5 Hz, 2H, NH—CH2—CH2), 2.96 (t, J=5.7 Hz, 2H, NH—CH2—CH2), 3.55 (s, 2H, NH—CH2—C), 3.82 (s, 3H, OCH3), 6.94~6.99 (m, 2H, 7-H, 9-H), 7.62 (d, J= 8.6 Hz, 1H, 10-H); IR (KBr) 波数υ: 3 440, 3 320, 2 920, 2 810, 1 690, 1 510, 1 280, 1 160, 1 040, 841, 781 cm-1; ESI-MS (质荷比m/z,%)为231.1 (M+, 5), 215.1 (25), 133.2 (13), 87.1 (42); C13H13NO3的元素分析实测值(计算值,%)为:C,67.29(67.52);H,5.35(5.67);N,6.31(6.06)。
2.2 3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮(Ⅱ)的表征
目标化合物(Ⅱ)为黄色固体,熔点为150~152 ℃;1H NMR (DMSO-d6, 500 MHz) δ为: 2.70 (t, J=5.4 Hz, 2H, N—CH2—CH2), 2.85 (t, J=5.2 Hz, 2H, N—CH2—CH2), 3.21 (s, 2H, N—CH2—Ar), 3.57 (s, 2H, N—
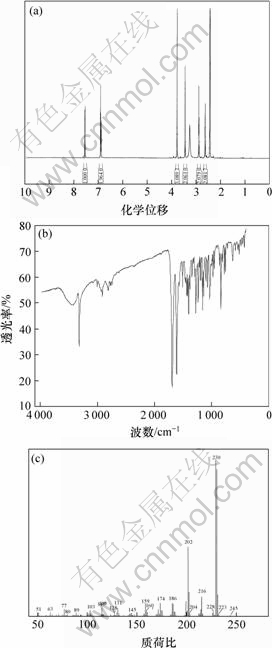
(a) 1H-NMR谱;(b) IR谱;(c) MS
图2 8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮的1H-NMR,IR和MS
Fig.2 1H-NMR, IR and MS of 8-methoxy-1, 2, 3, 4-tetrahydrochromeno[3, 4-c]pyridin-5-one

(a) 1H-NMR谱;(b) IR谱;(c) MS
图3 3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮的1H-NMR,IR和MS
Fig.3 1H-NMR, IR and MS of 3-(4-hydroxybenzyl)-8- methoxy-1, 2, 3, 4-tetrahydrochromeno [3, 4-c]pyridin-5-one
CH2—C), 3.85 (s, 3H, OCH3), 6.73 (d, J= 8.4 Hz, 2H, ArH), 6.92~6.99 (m, 2H, 7-H, 9-H), 7.13 (d, J=8.4 Hz, 2H, ArH), 7.61 (d, J=8.6 Hz, 1H, 10-H), 9.32 (s, 1H, OH); IR (KBr) υ: 3 400, 2 940, 1 690, 1 610, 1 510, 1 410, 1 280, 1 240, 1 160, 845, 820, 760 cm-1; ESI-MS (m/z,%)为338.2 (M+1+, 100), 339.2 (M+2+, 22); C20H19NO4的元素分析实测值(计算值,%)为:C,70.97(71.20);H,5.41 (5.68);N,3.93(4.15)。
3 结果与讨论
3.1 反应时间对分子间环加成反应的影响
反应时间对环加成反应的影响如表1所示。由表1可知,反应收率随反应时间的增加而逐渐增大,较适宜的时间为48 h,因为48 h后反应已基本完成。
表 1 反应时间对分子间环加成反应的影响
Table 1 Influence of reaction time on intermolecular cycloaddition reaction

注:反应温度为室温,n(3-甲氧基苯酚)?n(4-酮-3-甲酸甲酯哌啶盐酸盐)?n(硫酸)=n1?n2?n3= 2?2?60.
3.2 反应物配比对对分子间环加成反应的影响
3-甲氧基苯酚和4-酮-3-甲酸甲酯哌啶的分子间环加成反应需在强酸性条件进行。反应物的配比对该反应的影响见表2。由表2可知,随着反应物配比的增高,收率逐步增大。在选定的反应条件下,当n(3-甲氧基苯酚)?n(4-酮-3-甲酸甲酯哌啶盐酸盐)?n(硫酸)为2?5?120时,收率可达51.8%。考虑到这2种反应物的价格和收率,认为当它们的物质的量比为1?1时,即反应混合物配比为1?1?30时较好。
表 2 反应物配比对环加成反应的影响
Table 2 Influence of molar ratio of reactants on intermolecular cycloaddition reaction

注:反应温度为室温,反应时间为48 h.
3.3 反应时间对N-烷基化反应的影响
反应时间对N-烷基化反应的影响如表3所示。在室温下,当反应时间为30 h,n(Ⅰ)?n(4-羟基苯甲醛)?n(三乙酸基硼氢化钠)=n4?n5?n6=1?1?1.5时,N-烷基化反应的收率可达51.1%。由表3可知,目标化合物收率随反应时间的增加而逐渐增大,但反应时间超过20 h后,收率增加较小,故较适宜的反应时间为20 h。
表 3 反应时间对N-烷基化反应的影响
Table 3 Influence of reaction time on N-alkylation reaction

注:反应温度为室温,n(I)?n(4-羟基苯甲醛)?n(三乙酸基硼氢化钠) = n4?n5?n6 =1?1?1.5.
3.4 反应物配比对N-烷基化反应的影响
该N-烷基化反应需在乙酸存在下进行,反应物8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮(I)、4-羟基苯甲醛、三乙酸基硼氢化钠配比(n4?n5?n6)对N-烷基化反应影响较大,反应物的配比对该反应的影响见表4。由表4可知,随着反应物配比的增高,收率逐步增大。当n4?n5?n6为1?2?2.5,收率可达51.8%。
表 4 反应物配比对N-烷基化反应的影响
Table 4 Influence of molar ratio of reactants on N-alkylation reaction
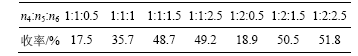
注:反应温度为室温,反应时间为20 h.
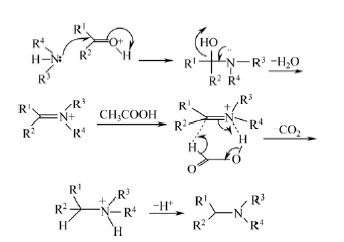
3.5 N-烷基化反应机理
在乙酸存在下,用1,3-二甲基-2-咪唑啉酮作疏质子溶剂、三乙酸基硼氢化钠作选择性还原试剂,4-羟基苯甲醛和8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮发生还原胺化得到目标化合物,其反应机理 如下:
4 结 论
a. 在强酸性条件下,通过3-甲氧基苯酚和4-酮-3-甲酸甲酯哌啶的分子间环加成反应,制备了中间体8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮。在此反应中,3-甲氧基苯酚中羟基间位的甲氧基作为供电子基团,对环加成反应是有利的。在室温下,当3-甲氧基苯酚、4-酮-3-甲酸甲酯哌啶盐酸盐、硫酸的投料比为 2:2:60时,反应48 h后收率可达49.2%。
b. 8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮通过N-烷基化反应即可得到目标产物3-(4-羟基苄基)-8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮。在室温下,当8-甲氧基-1, 2, 3, 4-四氢苯并吡喃[3, 4-c]吡啶-5-酮、4-羟基苯甲醛、三乙酸基硼氢化钠配比为1?2?2.5,反应20 h收率可达51.8%。
c. 该合成方法简单、反应条件温和。合成的化合物作为一种潜在的多巴胺D4受体拮抗剂。
参考文献:
[1] Jablensky A. Epidemiology of schizophrenia: the global burden of disease and disability[J]. Eur Arch Psychiatry Clin Neurosci, 2000, 250(6): 274-285.
[2] Seeman P. Antipsychotic drugs, dopamine receptors, and schizophrenia[J]. Clinical Neuroscience Research, 2001, 1(1): 53-60.
[3] Bailey J N, Breidenthal S E, Jorgensen M J, et al. The association of DRD4 and novelty seeking is found in a nonhuman primate model[J]. Psychiatric Genetics, 2007, 17(1): 23-27.
[4] Defagot M C, Falzone T L, Low M J, et al. Quantitative analysis of the dopamine D4 receptor in the mouse brain[J]. Neuroscience Research, 2000, 59: 202-208.
[5] Jürgen E, Harald H, Peter G. Cylic amidines as benzamide bioisosteres: EPC synthesis and SAR studies leading to the selective D4 receptor agonist FAUC 312[J]. Bioorganic & Medicinal Chemistry Letters, 2003, 13: 851-854.
[6] Mark H C, Christopher C G, Bonura M L, et al. Effectiveness of the selective D4 antagonist sonepiperazole in schizophrenia: A placebo-controlled trail[J]. Biological Psychiatry, 2004, 55: 445-451.
[7] TIAN Hai-bin, YIN Duan-zhi, ZHANG Lan, et al. Synthesis and biological evaluation of 3-{[4-[18F]fluorophenyl]methyl} piperazin-1-yl}methyl-1H-pyrrolo [2, 3-b]pyridine for in vivo studies of dopamine D4 receptor[J]. Journal of Radioanalytical and Nuclear Chemistry, 2004, 262(2): 383-389.
[8] LI Gu-cai, YIN Duan-zhi, WANG Ming-wei, et al. Syntheses of Two Potential Dopamine D4 Receptor Radioligands: 18F Labelled Chromeno[3, 4-c]pyridin- 5-ones[J]. Radiochim acta, 2006, 94(2): 119-122.
[9] Russell A G, Mechelle M L, Connie O V, et al. 8, 9-Dihydroxy- 1, 2, 3, 11b-tetrahydrochromeno[4, 3, 2-de]isoquinoline (dinoxyline), a high affinity and potent agonist at all dopamine receptor isoforms[J]. Bioorganic & Medicinal Chemistry, 2004, 12: 1403-1412.
[10] Staelens L, Oltenfreiter R, Blanckaert P, et al. In vivo evaluation of [123I]-3-(4-iodobenzyl)-8-hydroxy-1, 2, 3, 4-tetrahydro- chromeno [3, 4-c]pyridin-5-one: A presumed dopamine D4 receptor ligand for SPECT studies[J]. Nuclear Medicine and Biology, 2005, 32: 293-299.
[11] Brookes K, Xu X, Chen W, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: Association signals in DRD4, DAT1 and 16 other genes[J]. Molecular Psychiatry, 2006, 11: 934-953.
[12] Yukihide M, Yukari T, Ryo K, et al. Association between equine and polymorphisms in dopamine D4 receptor gene[J]. Mammalian Genone, 2005, 16: 538-544.
[13] Fuxe K, Canals M, Torvinen M, et al. Intramembrane receptor-receptor interactions: A novel principle in molecular medicine[J]. Journal of Neural Transmission, 2007, 114: 49-75.
[14] Mizuho N, Eiji H, Kazuo Y, et al. Association and synergistic interaction between promoter variants of the DRD4 gene in Japanese schizophrenics[J]. J Hum Genet, 2007, 52: 86-91.
[15] Paul C U, Thomas C, David T C, et al. Chromeno[3, 4-c] pyridin-5-ones: Selective human dopamine D4 receptor antagonists as potential antipsychotic agents[J]. J Med Chem, 1997, 40: 2688-2693.
[16] 李谷才, 尹端沚, 沈玉梅, 等. 苯并吡喃[3, 4-c]吡啶-5-酮类化合物的合成[J]. 有机化学, 2006, 26(6): 852-855.
LI Gu-cai, YIN Duan-zhi, SHEN Yu-mei, et al. Synthesis of chromeno[3, 4-c]pyridine-5-ones[J]. Chinese Journal of Organic Chemistry, 2006, 26(6): 852-855.
收稿日期:2007-03-16;修回日期:2007-05-08
基金项目:国家自然科学基金资助项目(30371634);2007年度湖南工程学院科研启动基金资助项目(0785)
作者简介:李谷才(1969-),男,湖南桃江人,博士,从事多巴胺D4受体分子探针研究
通信作者:李谷才,男,博士;电话:0732-8680393(O);E-mail: ligucai@163.com


