J. Cent. South Univ. (2017) 24: 789-795
DOI: 10.1007/s11771-017-3481-1
Selective separation of gallium from aluminum in SDS–Ga–Al and SDS–Ga–Al–fluoride systems by ion flotation
Bahri Zahra1, Rezai Bahram1, Kowsari Elaheh2
1. Department of Mining and Metallurgical Engineering, Amirkabir University of Technology, Tehran 158754413, Iran;
2. Department of Chemistry, Amirkabir University of Technology, Tehran 15916-34311, Iran
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract:
Selective separation of gallium from aluminum by ion flotation using sodium dodecyl sulfate (SDS) as an anionic surfactant and fluoride as an inorganic ligand was investigated. The experimental results were analyzed using the stability constants and speciation diagrams of fluoride metal complexes. The presence of fluoride in the solution has a positive influence upon the separation of gallium from aluminum. The results show that increasing the fluoride concentration makes a more effective separation of gallium from aluminum because of a simultaneous increase in the complexion of aluminum with fluoride and a change in the electrical charge of the aluminum  . The dehydration model of LIU and DOYLE was also applied to compare the ion flotation and the selectivity coefficients of gallium over aluminum with experimental results.
. The dehydration model of LIU and DOYLE was also applied to compare the ion flotation and the selectivity coefficients of gallium over aluminum with experimental results.
Key words:
ion flotation; gallium(III); selective separation; inorganic ligand;
1 Introduction
Gallium is in group 3A of the periodic table and is a trace-metallic element present in the Earth’s crust at approximately 16.9×10-6, which was discovered by Lecoq de Boisbaudran in 1875 [1]. It has wide applications in the electronics industry and medicine [2, 3]. It is mainly obtained as a byproduct from Bayer solutions of aluminum and from acidic solutions in the manufacture of zinc [4-6]. Owing to the increasing demand for gallium in recent years and its limited resource, the recovery of Ga(III) through a simple and effective method is necessary. For this reason, we have performed studies to develop Ga extraction from its impurity elements by ion flotation. In this study, because aluminum is as an impurity element in acidic solutions of gallium sources, the selective extraction of gallium from aluminum using SDS in the absence and presence of fluoride was tested by ion flotation. The ion flotation technique has a great number of advantages, including the selective removal of non surface-active ions from dilute (10-6–10-3 mol/L) aqueous solutions; low energy requirements; and environmental friendliness [7, 8]. LIU and DOYLE [9] suggested three strategies to manipulate the selectivity of ions of interest from mixtures of equivalent cations in ion flotation systems. One strategy was the use of a collector as a chelating agent; another strategy was the use of neutral ligands to change the effective diameter of one of the equivalent cations. The final strategy was the use of a non-surface active anion to change the electrical charge of the ions. Among published investigations on ion flotation, very few studies have been carried out using a non-surface active anion. JURKIEWICZ [10] reported the selective separation of Co-Cd, Zn-Cd and Co-Zn using thiocyanate as anionic complexes and cetyltrimethylammonium as a cation collector. WALKOWIAK and GRIEVES [11] studied the selective separation conditions of Zn, Cd, Hg, and Au cyanide complexes with the cationic surfactant hexadecyltrimethylammonium chloride using ion flotation. Their results showed that the selectivity sequence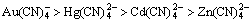 depended on the stability constants of the compounds formed. The effect of the halides F-, Cl-, Br- and I- on the selective separation and selectivity coefficients of Cd(II) from Zn(II) was studied using sodium dodecyl benzenesulfonate (DBSNa) and cetylpyridinium chloride (CPCl) by ULEWICZ et al [12]. They found that with higher stability constants for interactions between the Cd–halide complexes, more selective removal and higher selectivity coefficients of Cd were obtained using CPCl. The removal of Pb(II) from aqueous systems was studied using caffeic acid (CA) as collector by CRAIOVEANU et al [13]. Their results showed that the high separation efficiency (R=99.93%) obtained by ion and precipitate flotation. The removal of Cd from clean and Zn- and Cu-contaminated solutions was obtained to a small degree, i.e. 57%, 36% and 48%, respectively, using a rhamnolipid biosurfactant as the collector [14]. MAHMOUD et al [15] studied the removal of cadmium (II) from aqueous solution using potassium ethyl xanthate (KEtX) as a precipitating agent and sodium dodecyl sulfate (SDS) and hexadecyltrimethy- lammonium bromide (HDTMA) as collectors. The flotation of Ga (III) and Zn(II) from aqueous solutions showed that selective separation is possible by pH control [16]. Although the selective separation of mono- and divalent ions has been studied, no work has been published on the selective separation of gallium from aluminum in the presence of SDS and a non-surface active anion by ion flotation. Thus, the objective of this work was to study the effect of the process parameters i.e. pH and non-surface active anion concentration (fluoride concentration) on the selective separation of Ga(III) from Al(III) by ion flotation.
depended on the stability constants of the compounds formed. The effect of the halides F-, Cl-, Br- and I- on the selective separation and selectivity coefficients of Cd(II) from Zn(II) was studied using sodium dodecyl benzenesulfonate (DBSNa) and cetylpyridinium chloride (CPCl) by ULEWICZ et al [12]. They found that with higher stability constants for interactions between the Cd–halide complexes, more selective removal and higher selectivity coefficients of Cd were obtained using CPCl. The removal of Pb(II) from aqueous systems was studied using caffeic acid (CA) as collector by CRAIOVEANU et al [13]. Their results showed that the high separation efficiency (R=99.93%) obtained by ion and precipitate flotation. The removal of Cd from clean and Zn- and Cu-contaminated solutions was obtained to a small degree, i.e. 57%, 36% and 48%, respectively, using a rhamnolipid biosurfactant as the collector [14]. MAHMOUD et al [15] studied the removal of cadmium (II) from aqueous solution using potassium ethyl xanthate (KEtX) as a precipitating agent and sodium dodecyl sulfate (SDS) and hexadecyltrimethy- lammonium bromide (HDTMA) as collectors. The flotation of Ga (III) and Zn(II) from aqueous solutions showed that selective separation is possible by pH control [16]. Although the selective separation of mono- and divalent ions has been studied, no work has been published on the selective separation of gallium from aluminum in the presence of SDS and a non-surface active anion by ion flotation. Thus, the objective of this work was to study the effect of the process parameters i.e. pH and non-surface active anion concentration (fluoride concentration) on the selective separation of Ga(III) from Al(III) by ion flotation.
Four models have been assessed for the selectivity coefficient of competing counter ions in ion flotation operation:
A thermodynamic model was proposed to obtain the selectivity coefficient of one pair of metal ions with the same charge in the bulk solution by MORGAN et al [17, 18] according to the relation:
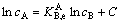 (1)
(1)
where cA and cB are the concentrations of two ions at different times during an ion flotation operation and C is a constant of integration, which depends on the initial solution conditions.
The selectivity coefficient corresponding to the charge exchange equilibrium at the interface was also calculated using [19]
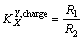 (2)
(2)
where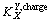 is the apparent charge selectivity coefficient, whereas R1 and R2 are the rates of removal of ions.
is the apparent charge selectivity coefficient, whereas R1 and R2 are the rates of removal of ions.
LIU and DOYLE developed a thermodynamic model using surface tension data for the determination of the selectivity coefficient, that is defined as [9]
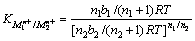 (3)
(3)
where n1 and n2 are valences of the ions, R is the gas constant and T is the absolute temperature. b1 and b2 are obtained from the slope of the surface tension plot of collector–ion solutions as a function of the collector concentration.
Another model was also developed based on the Gibbs free energy by LIU and DOYLE where the Gibbs free energy included different types of interactions (electrical interactions,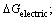 chelation,
chelation, 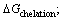 hydrogen bonding,
hydrogen bonding, 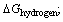 dehydration,
dehydration, 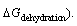 This model was expressed as [9]
This model was expressed as [9]
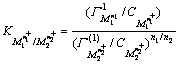 (4)
(4)
where  is concentration of ions and the adsorption density of ions
is concentration of ions and the adsorption density of ions 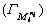 is expressed as
is expressed as
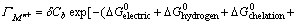
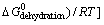 (5)
(5)
 (6)
(6)
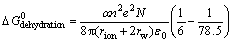 (7)
(7)
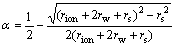 (8)
(8)
where δ is the thickness of the surface layer, rion is the crystal radius of the metal ion, rw the radius of a water molecule, N the Avogadro number, e the electronic charge, ε0 the permeability of a vacuum, n ions charge and α the fraction of the ion surface layer.
We applied LIU and DOYLE’s dehydration model for the ion flotation of gallium and aluminum with SDS. The selectivity coefficients predicted by LIU and DOYLE’s model and the apparent charge selectivity coefficients were compared in our experimental system.
2 Materials and methods
Gallium(III) nitrate hydrate 98+[Ga(NO3)3*xH2O] and aluminum chloride (AlCl3) were purchased from Merck. SDS (Merck, 98%) was used as the collector and the frother. Sodium fluoride (Merck, 99%) was used as an inorganic ligand. Caustic soda (NaOH) and hydrochloric acid (HCl), obtained from Merck, were used to adjust the solution pH. These reagents were prepared using double-distilled water and all solutions were used after 12 h. Ion flotation experiments were carried out in a Denver laboratory flotation machine with a 1 L capacity cell. The cell and all laboratory glassware were thoroughly rinsed with distilled water three times at the end of any test. The ion removal rate (R) was calculated according to the relationship [20]:
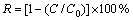 (9)
(9)
where C is the residual concentration of the metal ion in the treated solution and C0 is the initial concentration of the ion in the feed solution.
In the flotation tests, the initial concentrations of Ga and Al were the same and constant at 1.5×10-4 mol/L. Some operational and hydrodynamic parameters such as impeller speed, conditioning time, flotation time, liquid volume and SDS concentration were fixed at 1000 r/min, 3 min, 5 min, 1 L and 0.6×10-4 mol/L, respectively, during all experiments and the pH range of 0–5 was studied. Visual Minteq ver. 3.0 was used for modeling of gallium and aluminum speciation diagram.
3 Results and discussion
3.1 Effect of solution pH
Figures 1 and 2 show diagrams of the formed complex species for the Ga(III)–H2O and Al(III)–H2O systems in the absence and presence of 0.2 M fluoride as a function of pH. The formed complex species for the Ga(III)–fluoride system at 0
3, whereas the speciation diagram for the Al(III)–fluoride system at 0
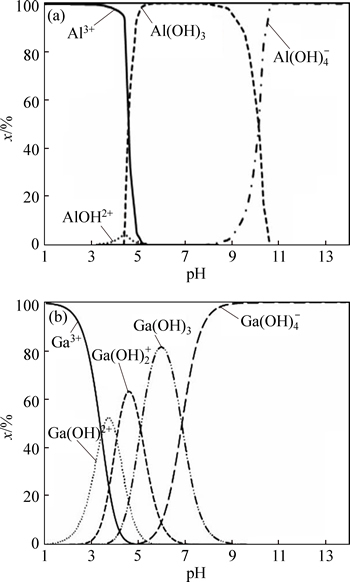
Fig. 1 Speciation diagram of gallium and aluminum species as function of pH
As can be seen from Table 1 and Fig. 2, the contributions of the complex species 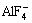 in Ga(III)– Al(III)–fluoride–H2O systems should be more predominant than GaF3 owing to the stronger Al(III)– fluoride complex. Therefore, to investigate the molar contributions of the formed complex species on the selective separation of gallium from aluminum, the effect of 0.2 mol/L fluoride on the percentage removal of ions was studied at different pH values.
in Ga(III)– Al(III)–fluoride–H2O systems should be more predominant than GaF3 owing to the stronger Al(III)– fluoride complex. Therefore, to investigate the molar contributions of the formed complex species on the selective separation of gallium from aluminum, the effect of 0.2 mol/L fluoride on the percentage removal of ions was studied at different pH values.
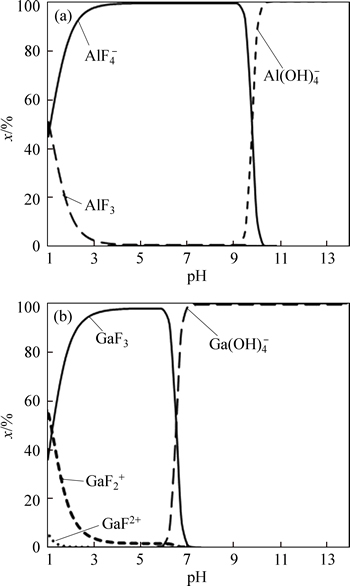
Fig. 2 Speciation diagram of gallium and aluminum species as function of pH in presence of 0.2 mol/L fluoride
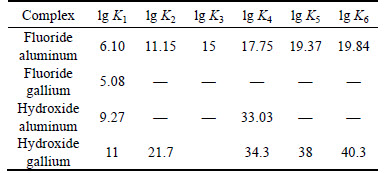
Table 1 Stability constant for metal complexes with inorganic ligands [21]
3.1.1 Effect of solution pH in SDS–Ga–Al systems
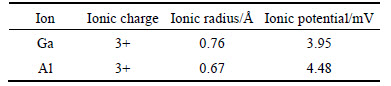
To investigate the effect of fluoride on the selective separation of gallium from aluminum, the Ga–Al–SDS flotation system was first studied. As can be seen from Fig. 3, the removal rate of Ga was more than that of Al when the pH value increases from 2 up to about 4, and was in good agreement with previous studies concerning ionic charge and ionic radius [22-24]. The main species in solution was Ga3+ and Al3+ in this pH range (2–4). According to previous studies, the selectivity sequence for adsorption and interaction of metal cations with anionic surfactants in ion flotation is related to the ionic charge and ionic radius of the metal cations [22-24]. A metal cation with a higher valency (higher ionic charge) and higher ionic radius is preferentially separated from the solution by anionic surfactants. The ionic charges, ionic radius, and ionic potentials (ratio between the ionic charge and the ionic radius) of Ga and Al are given in Table 2.
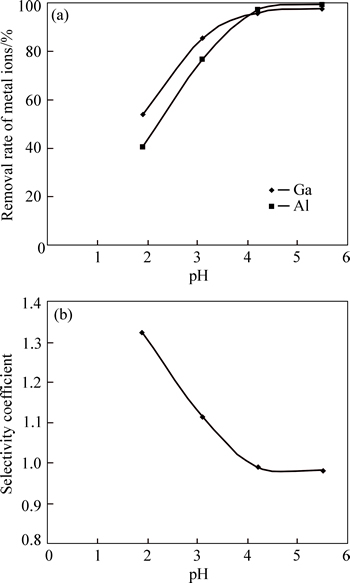
Fig. 3 Effect of pH on removal rate of Ga(III) and Al(III) in SDS–Ga–Al systems (a) and electivity coefficients of gallium over aluminum for different pH values (b)
Table 2 Ionic charges, ionic radii, and ionic potentials of Ga and Al
3.1.2 Effect of solution pH in SDS–Ga–Al–fluoride systems
The results of the ion flotation of gallium and aluminum versus pH from the solutions containing 1.5×10-4 mol/L of each metal and 0.2 mol/L of NaF are presented in Fig. 4.
The results indicated that with increasing pH the level of Ga(III) removal increased, but the selectivity coefficients of gallium over aluminum rapidly decreased. The removal rate of aluminum was magnitude lower than gallium at pH<4, owing to the formation of the complex 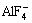 and decreasing interactions between aluminum and the anionic head group of SDS.
and decreasing interactions between aluminum and the anionic head group of SDS.
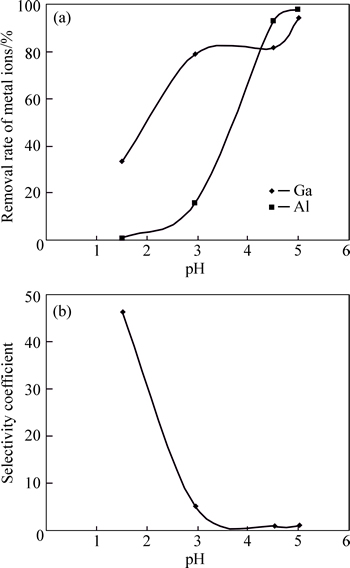
Fig. 4 Effect of pH on removal rate of Ga(III) and Al(III) in SDS–Ga–Al–fluoride systems (a) and selectivity coefficients of gallium over aluminum for different pH values (b)
As can be seen from Fig. 1, Al(OH)3 colloidal precipitate exists at pH values higher than 4. Therefore, a dramatic decrease of the selectivity coefficients of gallium over aluminum and an increase in Al(III) removal when the pH value was above 4 was related to the formation of more insoluble Al(OH)3 species and the occurrence of precipitate flotation. The solution pH was maintained at around 3 in the subsequent experiments. As can be seen from Figs. 3 and 4, fluoride significantly reduced the removal rate of aluminum and considerably enhanced the selectivity coefficients of gallium over aluminum, undoubtedly owing to Al3+–fluoride complexation and change in the surface charges of aluminum. This suggests that fluoride improved the selective separation of gallium from aluminum.
3.2 Influence of fluoride concentration
The complexation of ionic species with ligands and the species distributions depend on the nature of the metal ion, the concentration of the ion and the ligand and the solution conditions (i.e. pH, temperature) [25, 26]. Thus, an experimental investigation is presented on the selective separation of gallium from aluminum with respect to the fluoride concentration in the presence of an anionic surfactant using ion flotation. The concentration of the ions (Al(III) and Ga(III)), solution pH and temperature were constant at 1.5×10-4 mol/L, pH 3 and 25 °C respectively. The experimental results are presented in Fig. 5. It can be seen that the selective separation of gallium from aluminum increases with increasing fluoride concentration but the removal rate of gallium decreases. The decreasing level of Ga(III) removal with increasing fluoride concentration can be related to the increasing ionic strength whereby gallium ions have to compete for anionic surfactant with other ions, such as Na ions, in solution. The high selectivity coefficient of Ga(III)/Al(III) is explained in terms of increasing amounts of fluoride-aluminum complexes. With increasing fluoride concentration, the amounts of aluminum in the sublate (insoluble complexes formed in scum layer) decrease using SDS. The increase in the selective separation of gallium from aluminum with increasing fluoride concentration is explained in terms of increasing amounts of fluoride-aluminum complexes. Figure 6 shows speciation diagrams for Ga(III)–fluoride and Al(III)-fuoride systems in aqueous solutions as a function of the fluoride concentration using the stability constants listed in Table 1. The species distributions of fluoride complex species for gallium and aluminum versus the ligand concentrations were calculated using Visual Minteq ver. 3.0.
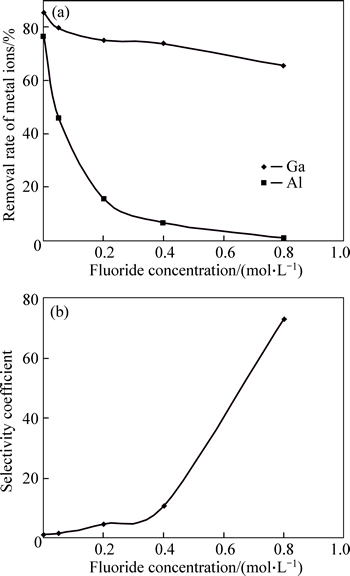
Fig. 5 Effect of fluoride concentration on removal rate of Ga(III) and Al(III) in SDS–Ga–Al–fluoride system (a) and selectivity coefficients of gallium over aluminum as function of fluoride concentration (b)
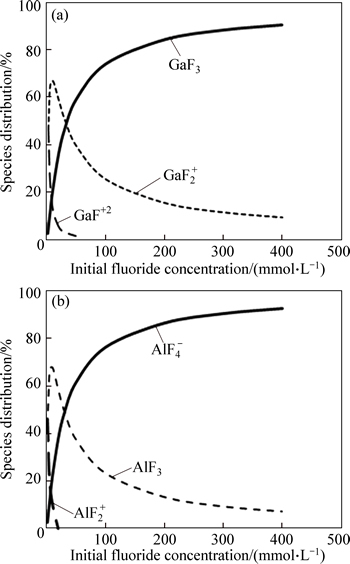
Fig. 6 Speciation diagrams of Al and Ga as function of fluoride concentration at total Ga concentration of 1.5×10-4 mol/L, initial solution pH 3, and temperature of 25 °C
It was observed that, with increasing fluoride concentration, the formation of 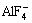 increased whereas GaF3 in the Ga(III)–fluoride system also increased. Therefore, aluminum should not be floated using an anionic collector at fluoride concentrations higher than 30 mmol/L, because of the change in the surface charges of aluminum with increasing fluoride concentration.
increased whereas GaF3 in the Ga(III)–fluoride system also increased. Therefore, aluminum should not be floated using an anionic collector at fluoride concentrations higher than 30 mmol/L, because of the change in the surface charges of aluminum with increasing fluoride concentration.
As can be seen from Table 1, the first aluminum- fluoride stability constants are greater than the first gallium-fluoride stability constant. Thus, complex formation between Al(III)-fluoride is stronger than Ga(III)-fluoride. As a result, Ga(III) recovery should be increased by using an anionic surfactant on the Ga(III)– Al(III)–fluoride–H2O systems in flotation, owing to stronger complexation of Al(III)-fluoride, the change in the surface charges of aluminum and increased electrostatic repulsion between aluminum and the anionic surfactant.
3.3 Comparison of selectivity coefficients predicted by dehydration model with experimental values
LIU and DOYLE [9] developed a dehydration model to predict the selectivity coefficients between ions with the same charge. We applied LIU and DOYLE’s model to compare the ion flotation of gallium and aluminum in the presence of SDS. To determine the selectivity coefficient between gallium and aluminum using LIU and DOYLE’s model, the solution pH was set at 1.9. Metal hydroxo complexes were negligible at this pH, thus the hydrogen bonding interaction was not considered. Sodium dodecyl sulfate (SDS) collector could not form chelates, therefore chelation interaction was not also considered. By canceling hydrogen bonding interaction, chelation interaction and electrical interaction, the selectivity coefficient of dehydration model for metal ions with the same charge by LIU and DOYLE has the relationship:
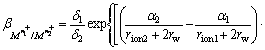
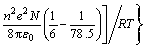 (9)
(9)
The calculated values of this model for Ga3+ and Al3+ are given in Table 3.
Table 3 Data and selectivity coefficients of LIU and DOYLE’s model for Ga3+ and Al3+
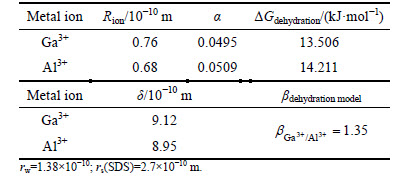
The selectivity coefficients predicted by LIU and DOYLE’s model and the experimental result were 1.35 and 1.32, respectively. Thus, we found that the selectivity coefficient predicted by LIU and DOYLE’s model agreed very well with our experimental result.
4 Conclusions
1) The results showed that selectivity coefficient of Ga3+ was higher than that of Al3+ using SDS at pH<4 in the ion flotation technique.
2) The results indicated that selective separation of gallium from aluminum using SDS by flotation was considerably increased in the presence of fluoride.
3) As a result, Ga(III) removal increases when using SDS in Ga(III)–Al(III)–fluoride–H2O systems by flotation, owing to stronger complexation of Al(III)– fluoride, the change in the surface charges of aluminum and increased electrostatic repulsion between aluminum and the anionic surfactant.
4) The values for Ga(III)/Al(III) selectivity coefficients increase with increasing fluoride concentration, owing to increasing amounts of fluoride– aluminum complexes.
5) The selectivity coefficients predicted by LIU and DOYLE’s model (1.35) and the experimental result (1.32) were in good agreement.
6) With further study, this method (ion flotation technique) can be used for the efficient selective separation of Ga(III)/Al(III) from acidic dilute solutions.
Acknowledgments
The authors would like to thank the Iran National Elites Foundation, Iranian Mines & Mining Industries Development & the Renovation and Geological Survey of Iran for financial support.
References
[1] MOSKALYK R R. Gallium: The backbone of the electronics industry [J]. Minerals Engineering, 2003, 16(10): 921-929.
[2] ZHAO Z, YANG Y, XIAO Y, FAN Y. Recovery of gallium from Bayer liquor: A review [J]. Hydrometallurgy, 2012, 125: 115-124.
[3] AHMED I M, EL-NADI Y A, EL-HEFNY N E. Extraction of gallium(III) from hydrochloric acid by Cyanex 923 and Cyanex 925 [J]. Hydrometallurgy, 2013, 131: 24-28.
[4] FLAMINI D O, SAIDMAN S B, BESSONE J B. Electrodeposition of gallium onto vitreous carbon [J]. Journal of Applied Electrochemistry, 2007, 37(4): 467-471.
[5] XU K, DENG T, LIU J, PENG W. Study on the recovery of gallium from phosphorus flue dust by leaching with spent sulfuric acid solution and precipitation [J]. Hydrometallurgy, 2007, 86(3): 172-177.
[6] DUMORTIER R, WEBER M E, VERA J H. Removal and recovery of gallium from aqueous solutions by complexation with sodium di-(n-octyl) phosphinate [J]. Hydrometallurgy, 2005, 76(3): 207-215.
[7] STOICA L, DINCULESCU M, PLAPCIANU C G. Mn(II) recovery from aqueous systems by flotation [J]. Water Research, 1998, 32(10): 3021-3030.
[8] SHAKIR K, GHONEIMY H F, BEHEIR S G, REFAAT M. Flotation of cesium coprecipitated with nickel hexacyanoferrate(II) from aqueous solutions and radioactive waste simulants [J]. Separation Science and Technology, 2007, 42(6): 1341-1365.
[9] LIU Z, DOYLE F M. A thermodynamic approach to ion flotation: II. Metal ion selectivity in the SDS–Cu–Ca and SDS–Cu–Pb systems [J]. Colloids Surfaces A: Physicochem and Engineering Aspects, 2000, 178(1): 93-103.
[10] JURKIEWICZ K. The removal of zinc from solutions by foam separation: I. Foam separation of complex zinc anions [J]. International Journal of Mineral Engineering, 1990, 28(3): 173-187.
[11] WALKOWIAK W, GRIEVES R B. Foam fractionation of cyanide complex of zinc(II), cadmium(II), mercury(II), and gold(III) [J]. Journal of Inorganic and Nuclear Chemistry, 1976, 38: 1351-1356.
[12] ULEWICZ M, WALKOWIAK W, KOZLOWSKI C. Selective flotation of zinc(II) and cadmium(II) ions from aqueous solutions in the presence of halides [J]. Physicochemical Problems of Mineral Processing, 2001, 35: 21-29.
[13] CRAIOVEANU G, STOICA L, CONSTANTIN C. Pb(II) removal from aqueous systems by flotation with novel collector [J]. Separation Science and Technology, 2015, 50(6): 802-812.
[14] BODAGH A, KHOSHDAST H, SHARAFI H, SHAHBANI ZAHIRI H, AKBARI NOGHABI K. Removal of cadmium(II) from aqueous solution by ion flotation using rhamnolipid biosurfactant as an ion collector [J]. Industrial & Engineering Chemistry Research, 2013, 52(10): 3910-3917.
[15] MAHMOUD M R, LAZARIDIS N K, MATIS K. Study of flotation conditions for cadmium(II) removal from aqueous solutions [J]. Process Safety and Environmental Protection, 2015, 94: 203-211.
[16] BAHRI Z, REZAI B, KOWSARI E. Selective separation of gallium from zinc using flotation: Effect of solution pH value and the separation mechanism [J]. Minerals Engineering, 2016, 86: 104-113.
[17] JMORGAN J D, NAPPER D H, WARR G G, NICOL S K. Measurement of the selective adsorption of ions at air/surfactant solution interfaces [J]. Langmuir, 1994, 10(3): 797-801.
[18] MORGAN J D, NAPPER D H, WARR G G. Thermodynamics of ion exchange selectivity at interfaces [J]. The Journal of Physical Chemistry, 1995, 99(23): 9458-9465.
[19] MICHEAU C, SCHNEIDER A, GIRARD L, BAUDUIN P. Evaluation of ion separation coefficients by foam flotation using a carboxylate surfactant [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 470: 52-59.
[20] EHRAMPOUSH M H, SALMANI M H, GHANEIAN M T, DAVOUDI M, FALLAHZADEH M H. Selectivity in removal of cadmium(II) from mixed metal effluents using ion flotation [J]. World Applied Science Journal, 2011, 13(1): 52-59.
[21] DEAN J A. Lange’s handbook of chemistry [M]. 12th Ed. New York: McGraw–Hill, 1978.
[22] SEBBA F. Ion flotation [M]. Elsevier Pub Co, 1962.
[23] LEMLICH R, AROD J. Adsorptive bubble separation techniques [M]. Academic Press, 1972.
[24] WALKOWIAK W. Mechanism of selective ion flotation: 1. Selective flotation of transition metal cations [J]. Separation Science and Technology, 1991, 26(4): 559-568.
[25] YOUNG S L, MATIJEVIC E. Precipitation phenomena of heavy metal soaps in aqueous solutions [J]. Journal of Colloid and Interface Science, 1977, 61(2): 287-301.
[26] AKANNI M S, OKOH E K, BURROWS H D, ELLIS H A. The thermal behaviour of divalent and higher valent metal soaps: A review [J]. Thermochimica Acta, 1992, 208: 1-41.
(Edited by YANG Bing)
Cite this article as:
Bahri Zahra, Rezai Bahram, Kowsari Elaheh. Selective separation of gallium from aluminum in SDS–Ga–Al and SDS–Ga–Al–Fluoride systems by ion flotation [J]. Journal of Central South University, 2017, 24(3): 789-795.
DOI:https://dx.doi.org/10.1007/s11771-017-3481-1Received date: 2015-12-10; Accepted date: 2016-04-11
Corresponding author: Rezai Bahram; Tel: +98-2166414999; E-mail: rezai@aut.ac.ir; b_rezai@hotmail.com
Abstract: Selective separation of gallium from aluminum by ion flotation using sodium dodecyl sulfate (SDS) as an anionic surfactant and fluoride as an inorganic ligand was investigated. The experimental results were analyzed using the stability constants and speciation diagrams of fluoride metal complexes. The presence of fluoride in the solution has a positive influence upon the separation of gallium from aluminum. The results show that increasing the fluoride concentration makes a more effective separation of gallium from aluminum because of a simultaneous increase in the complexion of aluminum with fluoride and a change in the electrical charge of the aluminum  . The dehydration model of LIU and DOYLE was also applied to compare the ion flotation and the selectivity coefficients of gallium over aluminum with experimental results.
. The dehydration model of LIU and DOYLE was also applied to compare the ion flotation and the selectivity coefficients of gallium over aluminum with experimental results.


