Trans. Nonferrous Met. Soc. China 29(2019) 2627-2637
Dissolution kinetics and removal mechanism of kaolinite in diasporic bauxite in alkali solution at atmospheric pressure
Yan WU, Xiao-lin PAN, Yue-jiao HAN, Hai-yan YU
School of Metallurgy, Northeastern University, Shenyang 110819, China
Received 29 March 2019; accepted 19 August 2019
Abstract:
A new chemical pre-desilication process of kaolinite in diasporic bauxite in alkali solution at atmospheric pressure was proposed. The dissolution kinetics and mechanism were studied by chemical analysis, XRD and SEM. The kinetic results of dissolution process show that the kaolinite is symbiotic with diaspore but without cladding. The dissolution ratio of kaolinite is close to 100% at 100 °C for 90 min. The dissolution kinetic equation is 1-(1-α)1/3=7.88×106exp[-64434/(RT)]t. With the low L/S (L/S= 10:1), the dissolution ratio of kaolinite decreases to 55%. This is due to the formation of lamellar hydroxyl—sodalite (OH—SOD) which is deposited on the surface of kaolinite and hinders the further dissolution of kaolinite. Under the optimum conditions, the A/S (mass ratio of Al2O3 to SiO2) of dissolved residues is increased to 8.55, while the A/S of the bauxite is only 4.97.
Key words:
Bayer process; kaolinite; pre-desilication; dissolution kinetics; sodalite;
1 Introduction
Bauxite is the main raw material of the alumina production industry, especially the main source of aluminum metal industry [1]. Depending on the difference of main alumina-bearing minerals, bauxites can be classified into three categories: gibbsitic bauxite (Al(OH)3), boehmitic bauxite (γ-AlO(OH)) and diasporic bauxite (α-AlO(OH)). Other than that, there are other mineral components in bauxite such as kaolinite (Al2Si2O5(OH)4), quartz (SiO2), hematite (Fe2O3), goethite (FeO(OH)), rutile/anatase (TiO2), calcite (CaCO3) and other impurities in minor or trace amounts [2,3].
Bayer process is the principal method to produce alumina from bauxite worldwide. In Bayer process, kaolinite is also dissolved with the digestion of the alumina hydrate-containing ores in caustic solution, resulting in a large amount of SiO2 entering the solution. The presence of SiO2 dissolved in Bayer liquors results in the formation of undesirable sodium aluminosilicate accumulated in the heat exchangers used in alumina refining plants, which is commonly known as desilication product (DSP) [4,5]. The formation of DSP not only causes the loss of aluminum and sodium in the solution, but also results in a pressure drop, flow restriction and energy consumption as well as serious fouling in the heat exchangers [5,6].
Several processes such as floating process [7,8], lime process [9-14], and roast-leach process have been developed and applied to producing alumina from low- grade bauxite ore in the alumina refineries [14-16]. The flotation-Bayer process is the most widely used method for physical pre-desilication, but is generates more tailings simultaneously (25% of the initial bauxite ores in mass fraction) [17]. The lime Bayer process cannot effectively reduce the scale, and cannot treat low-grade bauxite with A/S<5 [18]. The roast-leach process has attracted more and more attention in recent years. This process can be described as the decomposition of kaolinite by roasting process, and formation of soluble silicon oxide and insoluble aluminum oxide. Then, the silicon oxide is dissolved in alkali solution to desilication. The desilicated bauxite is then used in the Bayer process with variation capable of digesting the alumina produced after being roasted [19-22]. During the roasting process, the stability of alumina in kaolinite, gibbsite and boehmite is increased. Therefore, it is more difficult for the roasted bauxite to be digested by the normal Bayer process. In order to solve this problem, the roasting process of bauxite has been studied in detail, and the digestion process of roasted products has also been discussed [23-27]. In addition, the effect of silica in roasted products on the digestion process was also discussed [22,28].
Kaolinite is 1:1 type phyllosilicate mineral, which is composed of tetrahedral SiO4 sheet and octahedral AlO2(OH)4 sheet in each layer [29]. The hexagonal grid arrangement of tetrahedral SiO4 in lattice of kaolinite makes a pseudo-hexagonal lamellar morphology appear in the microcrystalline of kaolinite. The dissolution mechanism of kaolinite is regarded as a three-stage process: kaolinite firstly is dissolved in alkali solution in the form of soluble silicate and aluminate, then the silicate in the alkaline solution reacts with the aluminate to form a metastable aluminosilicate. Finally, the aluminosilicate was precipitated when the concentration of silica exceeds its equilibrium concentration [30]. The reactive silica dissolution and stabilization of the active silica in concentrated NaOH-NaAl(OH)4 solutions were investigated. Two distinct steps: active silica dissolution and DSP precipitation, control the whole leaching process of silica in NaOH-NaAl(OH)4 media [31].
Based on the above analyses of kaolinite, transferring a large proportion of the kaolinite from bauxite directly into solution without roasting activation will be crucial for this process. In this work, a new chemical pre-desilication process for kaolinite-rich diasporic bauxite was proposed and investigated in detail. Under the atmospheric pressure, kaolinite can be dissolved by alkali solution, yet the diaspore cannot be dissolved so that the purpose of improving the A/S of concentrate can be achieved with very simple treatment steps: alkali dissolution and liquid-solid separation. The separated solution can be used to prepare zeolite products under certain conditions. Previous studies have shown that the solubility and dissolution ratio of kaolinite in alkali solution were strongly related to the dissolution conditions and the morphology of kaolinite. However, there is no detailed study on the dissolution process of kaolinite-rich diasporic bauxite in alkali solution at atmospheric pressure. This study aims to better understand the dissolution process of kaolinite at the atmosphere pressure in alkaline solution. This kind of kaolinite is associated with diaspore in bauxite. Characteristic of the dissolved residues was also studied to reveal reaction mechanisms. The final effect of the new chemical pre-desilication process would be determined through the A/S in the solid phase before and after dissolution.
2 Experimental
Analytical-grade NaOH (Tianjin Kermel Chemical Reagent Co., Ltd.) was used for preparation of alkali solution with the purity of 98.0%. Unless otherwise stated, all chemicals used in this experiment were analytical reagent grade and all solutions in this experiment were prepared by deionized water.
In this experiment, kaolinite-rich diasporic bauxite from Shanxi province in China was studied after grounding to a particle size of less than 250 μm in diameter. All dissolution experiments were performed in a water bath heating device. Four-mouth flasks with a capacity of 500 mL were used and the condenser, agitation, thermocouple and feed switch were placed on four mouths, respectively. The whole dissolution process included dissolution, filtration separation, water washing of the dissolved residues and then drying at 90 °C. For each dissolution experiment, 300 mL of alkali solution with Na2O concentration of 230 g/L was heated to a certain temperature, and then a certain mass of kaolinite-rich diasporic bauxite was put into the alkali solution and dissolved for a certain time. After the dissolution reaction, the residue was filtrated and the filter cake was washed by hot deionized water to a pH value of 7. The concentrations of Na2O, Al2O3 and SiO2 in filter solution and the contents of Na2O, Al2O3 and SiO2 of dissolved residues were analyzed.
The phase analysis was determined by X-ray powder diffraction (XRD, PANalytical PW3040/60) using Cu Kα irradiation (λ=1.5406 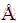 ) at 40 kV with a scan speed of 0.05 (°)/s over 2θ angle range of 5°-90°. The 2014 PDF database from BRUKER was used for reflection identification. The morphology analysis was observed by scanning electron microscopy (SEM, SHIMADZU SSX-550) and energy-dispersive X-ray spectroscopy (EDS, DX-4). The samples were gold-coated before SEM-EDS analysis. The X-ray fluorescence analysis (XRF) instrument was applied to determining the chemical composition of bauxite and dissolved residues.
) at 40 kV with a scan speed of 0.05 (°)/s over 2θ angle range of 5°-90°. The 2014 PDF database from BRUKER was used for reflection identification. The morphology analysis was observed by scanning electron microscopy (SEM, SHIMADZU SSX-550) and energy-dispersive X-ray spectroscopy (EDS, DX-4). The samples were gold-coated before SEM-EDS analysis. The X-ray fluorescence analysis (XRF) instrument was applied to determining the chemical composition of bauxite and dissolved residues.
The concentrations of Al2O3 and Na2O in solution were analyzed by the volumetric method, and the concentration of SiO2 was analyzed on the basis of silicon-molybdenum blue spectrophotometry by a 722S spectrophotometer.
3 Results and discussion
3.1 Mineral analysis of kaolinite-rich diasporic bauxite
The chemical composition of kaolinite-rich diasporic bauxite is shown in Table 1, and the mineral composition is shown in Fig. 1. Diaspore and kaolinite are the main mineral forms of alumina. In addition, anatase is the mineral form of titanium dioxide and calcite is the mineral form of calcium oxide. There is no quartz detected by XRD in kaolinite-rich diasporic bauxite, so all of the SiO2 are in the form of kaolinite in this bauxite. The calculated mineral composition is shown in Table 2. The A/S of the bauxite is 4.97, which is not suitable for digestion with the Bayer process.
Table 1 Chemical composition of kaolinite-rich diasporic bauxite (wt.%)

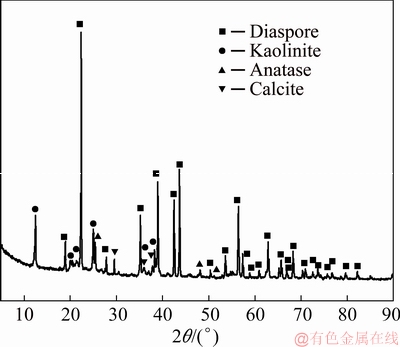
Fig. 1 XRD pattern of kaolinite-rich diasporic bauxite
Table 2 Mineralogical composition of kaolinite-rich diasporic bauxite (wt.%)

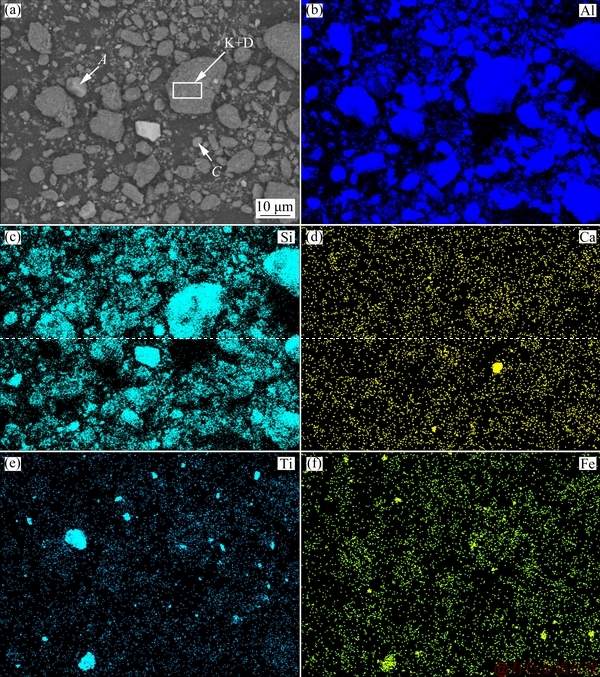
Fig. 2 SEM image of kaolinite-rich diasporic bauxite (a) and scanning maps (b)-(f)
Figure 2(a) shows the microstructure of the kaolinite-rich diasporic bauxite. The map scanning results of Al, Si, Ca, Ti and Fe elements are shown in Figs. 2(b-f), respectively, and then several typical phases in Fig. 2(a) are determined. The point of A in Fig. 2(a) indicates that the particle is anatase with massive structure and smooth surface. The crystallization is more complete and the size is more uniform. Iron is scattered in bauxite by permeating in kaolinite and diaspore, and the dense iron phases are symbiotic with anatase, as shown in Fig. 2(e). The point of C in Fig. 2(a) indicates that the particle is calcite. The kaolinite (short for K) is symbiotic with diaspore (short for D), as shown in area point by K+D. Figure 3(a) shows the magnified micromorphology of the square area in Fig. 2(a). A mixture phase of kaolinite and diaspore was formed because some of flake crystals covered on the surface of diaspore, as shown in Fig. 3(a). Kaolinite is a plate structure and it is stacked by layered crystals. The regional EDS analysis is shown in Fig. 3(b).
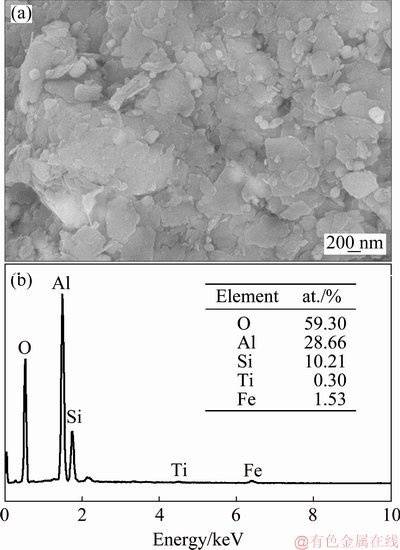
Fig. 3 Magnified micromorphology of particles K+D (a) and EDS analysis (b)
3.2 Dissolution kinetics of kaolinite in diasporic bauxite
The dissolution process of kaolinite in alkali solution is a liquid-solid reaction. A solution containing 2 g/L of SiO2 and 2 g/L of Al2O3 was stirred for 5 h under the condition of 10 g/L seed at 90 °C, and the composition of the solution remained unchanged. Therefore, to avoid the formation of DSP, the concentration of 2 g/L SiO2 was determined as the maximum value, so the liquid-to-solid ratio (short for L/S) of 70:1 was adopted. The dissolution kinetics of kaolinite was calculated according to the concentration of SiO2 in solution, which was shown in Fig. 4(a). Three residues dissolved at 90, 95 and 100 °C for 120 min were analyzed by XRD and shown in Fig. 4(b). No DSP peak was found in the three residues. This indicated that no secondary reaction occurred under the L/S of 70:1.
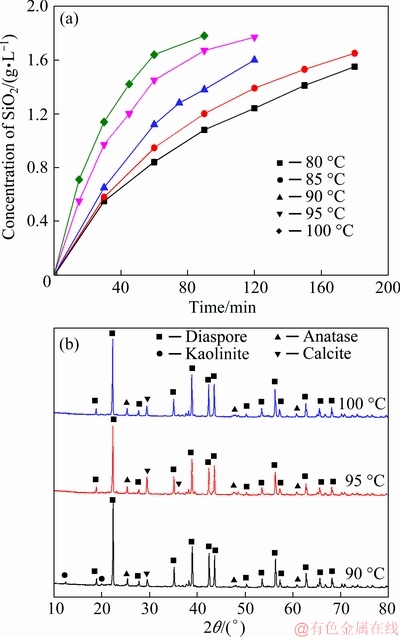
Fig. 4 Concentration of SiO2 in solution (a) and XRD patterns of three dissolved residues (b)
When kaolinite entered the solution, it reacted with the alkali in the solution to form aqueous silicate and aluminate species, as shown in formula (1). Chemical thermodynamic calculations indicated that Na2SiO3 was the main solution silicate specie and NaAlO2 was the main aluminate specie [28].
Al2O3·2SiO2·2H2O+6NaOH→2NaAlO2+2Na2SiO3+5H2O (1)
The dissolution ratio of SiO2 is expressed by α and calculated by Eq. (2), as follows:
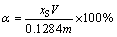 (2)
(2)
where xS is the mass concentration of SiO2 in dissolution solution (g/L); V is the volume of dissolution solution (L); m is the total mass of kaolinite-rich diasporic bauxite of input (g).
Under a certain L/S, the relationship between α and time was consistent with the relationship between xS and time in Fig. 4(a). With the increase of dissolution temperature, the initial dissolution ratio of SiO2 increased and the time to reach equilibrium shortened. This indicated that the higher dissolution temperature promoted the dissolution of SiO2.
The dissolution reaction was carried out in three steps. First, the liquid reactant diffused to the solid surface due to the concentration gradient in solution. It is usually called an external diffusion process. The second step was the reaction at the solid-liquid interface. The final step was to diffuse the product from the interface to the liquid through external diffusion [32].
When the dissolution process was controlled by chemical reaction or external diffusion, the dissolution kinetics equation was as follows:
1-(1-α)1/3=kt (3)
where α (%) is the dissolution ratio of SiO2 at the dissolution time of t and k is the apparent rate constant.
However, the raw material in this experiment was kaolinite-rich diasporic bauxite, and kaolinite was associated with diaspore. The dissolution process of kaolinite in diasporic bauxite was different from the pure kaolinite and it was affected by other minerals in bauxite. The presence of other components could affect the dissolution process of kaolinite, so the diffusion process becomes the restrictive step of the whole reaction. If there is no new phase generated, the dissolution kinetics equation can be described by the shrinking unreacted core model as follows:
1-2/3α-(1-α)2/3=kt (4)
The calculated dissolution ratios of SiO2 were substituted into Eqs. (3) and (4), the linear relationships obtained by fitting Eqs. (3) and (4) were shown in Fig. 5. The bigger the R2 is, the better the model fits the data. The results showed that the linear relationships between 1-(1-α)1/3 and t were better than those between 1-2/3α-(1-α)2/3 and t. In this experiment, stirring method was used to reduce the influence of external diffusion. This indicated that the dissolution ratio was mainly affected by reaction at the solid-liquid interface.
The apparent activity energy was calculated by apparent rate constants at different temperatures. The dissolution kinetics equation of kaolinite in diasporic bauxite was obtained from the Arrhenius equation as follows:
ln k=-E/(RT)+ln A (5)
where E is the activity energy (kJ/mol), R is the mole gas constant (8.314 J/(mol·K)), T is the dissolution temperature (K) and A is the pre-exponential factor.
According to k values at different temperatures, the Arrhenius curve of the dissolution process was obtained and shown in Fig. 6.
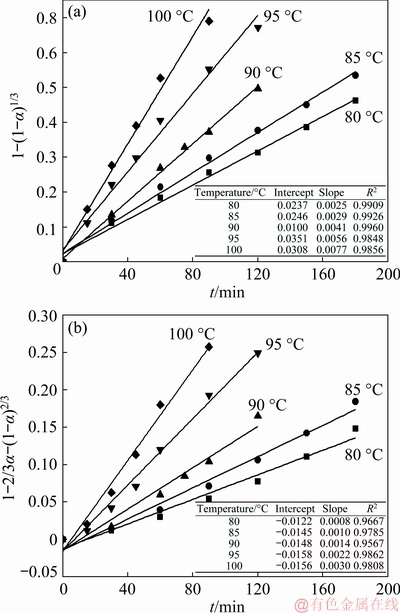
Fig. 5 Linear relationships of 1-(1-α)1/3 vs t (a) and 1-2/3α-(1-α)2/3 vs t (b) of kaolinite in bauxite at different temperatures
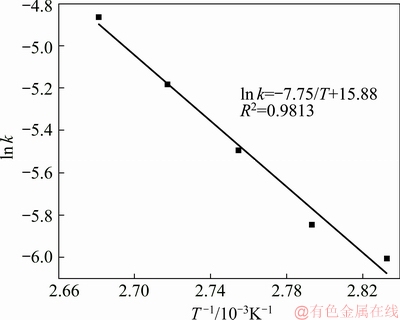
Fig. 6 Arrhenius curve of dissolution of kaolinite
As shown in Fig. 6, the line slope was -7.75 and intercept was 15.88. The activity energy of the reaction calculated by the Arrhenius equation (Eq. (5)) was 64.434 kJ/mol. According to the above discussion, for kaolinite in diasporic bauxite, the dissolution kinetic equation in alkali solution is expressed as
1-(1-α)1/3=7.88×106exp[-64434/(RT)]t (6)
The result showed that the dissolution activity energy of the kaolinite-rich diasporic bauxite in alkali solution was 42-800 kJ/mol, which belonged to chemical reaction control. ROACH and WHITE [33] studied the dissolution kinetics of kaolinite in sodium aluminate solution, suggesting that the reaction rate of kaolinite dissolution was influenced by crystalline form and surface area. The activity energy of compact kaolinite dissolution in sodium aluminate solution was 99 kJ/mol, and the interfacial chemical reaction was a restrictive step.
3.3 Reaction mechanism of kaolinite-rich diasporic bauxite in alkali solution
The above kinetic results were concerned with the dissolution reaction of kaolinite, so the L/S of the dissolution process was high enough without causing any secondary reaction. Bauxite refinery usually adopts lower L/S to reduce operating costs. However, in this experiment, a low L/S will lead to the formation of a large amount of DSP, which will reduce the pre-desilication effect and the A/S ratio of dissolved residues. Therefore, the L/S of 10:1 (100 g/L) was adopted and studied in detail, which could not cause the formation of a large amount of DSP, but also could be accepted by industrial production. In the dissolution process of kaolinite, the formation reaction of DSP could be described according to the chemical reaction (7) [4].
The formation of DSP in alkali solution:
xNa2SiO3(aq)+2NaAlO2(aq)+4H2O→Na2O·Al2O3·xSiO2·(4-x)H2O(s)+2xNaOH(aq) (7)
With the dissolution of kaolinite, the formation of DSP was carried out simultaneously. The two chemical reactions were coinstantaneous in alkali solution. In fact, the DSP formed under different conditions varied greatly in compositions and structures. These DSPs were mainly in the forms of sodalite (SOD), zeolite (ZEO) and cancrinite (CAN) [26]. The zeolite (ZEO) and sodalite have the same cubic sodalite-structure, while cancrinite has a hexagonal structure [34].
Figure 7 shows the equilibrium phase diagram of Na2O-A12O3-SiO2-H2O system at 80 °C (where the solubility of SiO2 is ignored). It shows the equilibrium solids of formation in the solution with different concentrations of A12O3 and Na2O. The equilibrium solid phase formation in solution with higher alkali concentration and higher molar concentration ratio of Na2O to A12O3 in solution (MR) is alkaline sodalite or hydroxyl—sodalite (OH—SOD) [2]. According to the above analyses, the DSP formed in alkali solution with the Na2O concentration of 230g/L was hydroxyl—sodalite, with the molecular formula of 3(Na2O·A12O3·2SiO2)·2NaOH·2H2O.
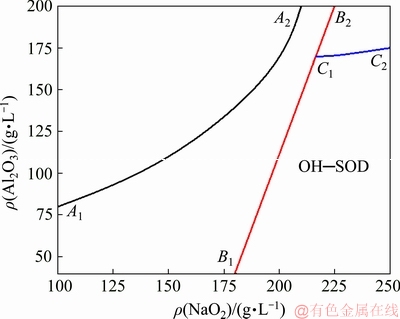
Fig. 7 Equilibrium phase diagram of Na2O-A12O3-SiO2-H2O system at 80 °C [2]
With L/S of 10:1, the single factor experiments of temperature and time were carried out. Chemical compositions of dissolved residues at different reaction temperatures and reaction time were shown in Table 3. Al2O3 in residues mainly existed in three phases: OH—SOD, remnant kaolinite and non-reactive diaspore,
and the contents were expressed by 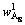 ,
, 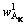 and
and  respectively. According to the chemical composition of the dissolved residues, the contents of Al2O3 in each phase of residues were calculated by Eqs. (8)-(10), the contents of DSP in residues were calculated from the chemical formula of OH—SOD, and all results were listed in Table 3.
respectively. According to the chemical composition of the dissolved residues, the contents of Al2O3 in each phase of residues were calculated by Eqs. (8)-(10), the contents of DSP in residues were calculated from the chemical formula of OH—SOD, and all results were listed in Table 3.
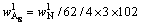 (8)
(8)
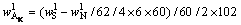 (9)
(9)
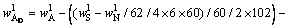
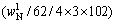 (10)
(10)
where 102, 60 and 62 are relative molecular mass of Al2O3, SiO2 and Na2O respectively;  ,
, 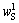 and
and 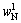 are the contents of Al2O3, SiO2 and Na2O in residues, respectively. The DSP chemical formula was determined from OH—SOD as 3Na2O·3Al2O3·6SiO2· 2NaOH·2H2O.
are the contents of Al2O3, SiO2 and Na2O in residues, respectively. The DSP chemical formula was determined from OH—SOD as 3Na2O·3Al2O3·6SiO2· 2NaOH·2H2O.
The content of Al2O3 in diaspore was considered as an internal standard because of its insolubility in alkali solution at atmospheric pressure. The dissolution ratio of kaolinite (δK) and the formation ratio of DSP (δS) were calculated by Eqs. (11) and (12), and the results are shown in Fig. 8.
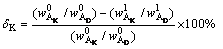 (11)
(11)
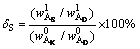 (12)
(12)
The Al2O3 in bauxite mainly existed in diaspore and kaolinite, and the contents were expressed by  and
and 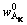 , and the values were calculated from Table 2.
, and the values were calculated from Table 2.
Table 3 Chemical compositions of dissolved residues at different temperatures for different time (wt.%)

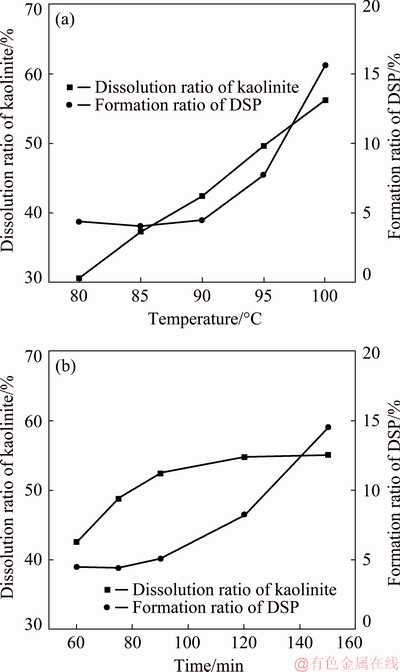
Fig. 8 Dissolution results at different temperatures for 60 min (a) and at 90 °C for different time (b)
As shown in Fig. 8(a), the dissolution ratio of kaolinite continuously increased with the increasing of dissolution temperature from 80 to 100 °C, which was consistent with the kinetic analysis. However, with the increasing of temperature, the formation ratio of DSP also increased, especially when the temperature exceeded 90 °C. Although the increasing of temperature was beneficial to the dissolution of kaolinite, it also increased the formation of DSP. In order to improve the A/S of dissolved residues, suitable temperature of dissolution should be selected.
To determine the influence of dissolution time on dissolution result, single factor experiments of dissolution were carried out at 90 °C, as shown in Fig. 8(b). It showed that the dissolution ratio of kaolinite increased with the prolongation of dissolution time, and then tended to be stable. When the dissolution time exceeded 120 min the dissolution ratio of kaolinite remained stable. The precipitation ratio of DSP slowly increased with the increasing of dissolution time. When the dissolution time exceeded 120 min, the formation ratio of DSP increased rapidly.
The XRD patterns of dissolved residues at different temperatures are shown in Fig. 9(a). With the increase of reaction temperature, the characteristic peak intensity of kaolinite decreased. This indicated that the contents of kaolinite in the dissolved residues decreased.
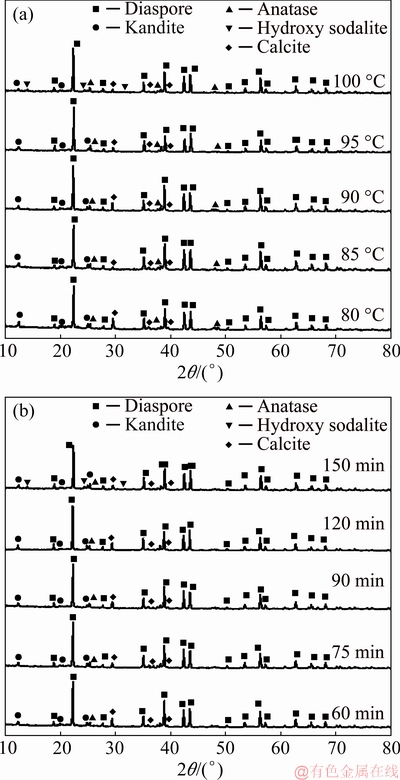
Fig. 9 XRD patterns of dissolved residue at different temperatures for 60 min (a) and at 90 °C for different time (b)
When the reaction temperature reached 100 °C, the DSP was found in dissolved residues. This indicated that the increasing of temperature promoted the formation of DSP. Figure 9(b) shows the XRD patterns of dissolved residues for different time, and DSP was detected in the dissolved residue with the reaction time of 150 min. The chemical composition (see Table 3) of the dissolved residues showed that the DSP precipitated into the dissolved residues, but the content of DSP was not enough to be analyzed by XRD when the dissolution time was less than 120 min. The dissolution results at different temperatures and time showed that the dissolution of kaolinite and the formation of DSP were simultaneous. When the content of Na2O in the dissolved residues exceeded 5%, the characteristic peak of DSP in dissolved residues could be detected by XRD. So the DSP phase was found only in the residues dissolved at 100 °C for 60 min and at 95 °C for 150 min.
From the XRD patterns of the dissolved residues, the crystallinity of a crystal plane was obtained by Jade software. According to the crystallinity of main crystal plane of kaolinite in dissolved residues, the solubility of crystal plane was calculated by Eq. (13) as follows:
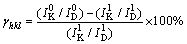 (13)
(13)
where γhkl is the solubility of crystal plane of kaolinite; 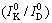 and
and 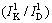 are the relative crystallinity of a crystal plane of kaolinite in raw materials
are the relative crystallinity of a crystal plane of kaolinite in raw materials 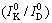 and in dissolved residues
and in dissolved residues 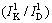 , respectively.
, respectively.
Figures 10 (a) and (b) showed the effect of time and temperature on the solubility of (001) and (110) crystal planes of kaolinite, respectively. It showed that the solubility of the (001) crystal plane was much higher than that of the (110) crystal plane. This indicated that the (001) crystal plane was more likely to been destroyed than the (110) crystal plane by alkali. With the increase of reaction temperature, the solubility of the (001) crystal plane increased gradually. The results also indicated that the dissolution of kaolinite was promoted by increasing temperature.
The fundamental structure unit of kaolinite is composed of a plane of tetrahedral SiO4 linked by oxygen atoms parallel to a plane of octahedral AlO2(OH)4. The hexagonal grid arrangement of tetrahedral SiO4 in the lattice of kaolinite makes a pseudo-hexagonal lamellar morphology appearing in the microcrystalline of kaolinite (see Fig. 1). In general, for kaolinite, there are main basal planes at (001) with 2θ=12.5°, (002) with 2θ=24.8° and also the prism planes at (020) with 2θ=19.9°, (110) with 2θ=21.2° (Reference code 00-003-0052). For a hypothetical kaolinite crystal, if the (001) crystal plane is defined as a horizontal crystal surface, it can be seen as the horizontal extension of a plane of tetrahedral SiO4 or a plane of octahedral AlO2(OH)4. The (110) crystal plane can be seen as the connection plane of the tetrahedral SiO4 and octahedral AlO2(OH)4 with H-bond as a force. It should be noted that the calculating of solubility of (110) plane may be affected by (020) plane because they are close to each other. In this work, this effect was neglected, because they were prismatic planes, more deeply research is still under way. The solubility of the crystal planes in Fig. 10 showed that the dissolution process of kaolinite in alkali solution was carried out by the way of peeling damage on crystal planes and insertion damage in interlayer synchronously. In addition, the peeling damage on crystal planes exceeded insertion damage in interlayer.
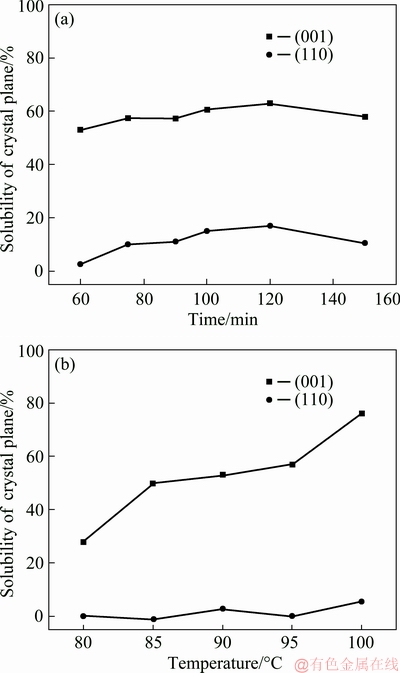
Fig. 10 Solubility of crystal plane of kaolinite at 90 °C for different time (a) and at different temperatures for 60 min (b)
The SEM image of the dissolved residue was shown in Fig. 11, and the particle size in dissolved residue was smaller than that of raw material (Fig. 2(a)).
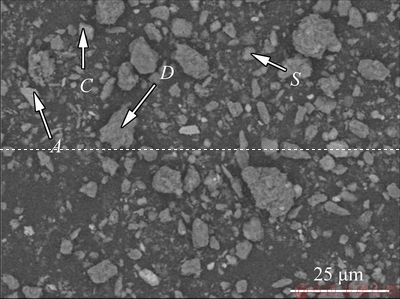
Fig. 11 SEM image of dissolved residue at 90 °C for 120 min
In Fig. 11, four typical particles of A, C, D and S were selected for detailed observation and composition analysis. The results of micromorphology and EDS analysis of these particles were shown in Figs. 12(a)-(h) respectively. According to the results of EDS analysis, it was determined that the composition of point A was anatase associated with kaolinite and diaspore. The composition of point C was calcite. The composition of point D was diaspore with a small amount of kaolinite. The composition of S was OH—SOD associated with kaolinite.
Figure 12(g) showed that the crystal particle of DSP was attached to kaolinite. It was observed that lamellar crystals interlaced each other and combined in the form of edge-face, and then grew on kaolinite body. In the dissolution process by alkali solution, kaolinite structure was broken and has a large number of defects with high surface energy. These fresh defects promoted the nucleation process of DSP and precipitation reaction.
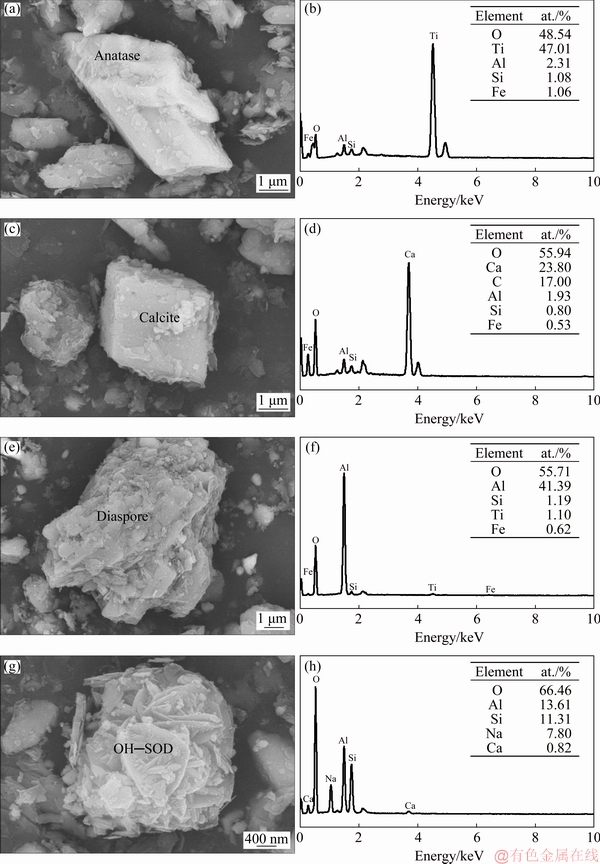
Fig. 12 Micromorphologies of dissolved residue (a, c, e, g) and corresponding EDS analyses (b, d, f, h) of point A (a, b), point C (b, d), point D (e, f) and point S (g, h) in Fig. 11
DSP precipitated on the surface of kaolinite, and resulted in wrapping and covering the kaolinite surface. The precipitation of DSP made the dissolution process of kaolinite more difficult. The kinetic studies showed that the increase of temperature promoted the dissolution of kaolinite, but also promoted the formation of DSP. So that SiO2 was returned to the dissolved residues and the A/S of the residues was decreased. Above analysis was confirmed by the results of single factor experiments, as shown in Fig. 8. The morphology of precipitates was consistent with Ref. [28]. The lattice parameters of hydroxyl-sodalite precipitated in this experiment agreed with those in Ref. [35]. The d-spacings in lattice plane of (211) and (110) were 0.3634 and 0.6275 nm respectively, which were 0.3633 and 0.6275 nm.
4 Conclusions
(1) The dissolution kinetics of kaolinite-rich diasporic bauxite in alkali solution at atmospheric pressure was studied. The results show that the dissolution process of kaolinite is mainly controlled by chemical reaction, and the effective way to improve the dissolution ratio is to increase the temperature. The dissolution kinetic equation is 1-(1-α)1/3=7.88×106× exp[-64434/(RT)]t. The dissolution ratio of kaolinite is close to 100% at 100 °C for 90 min with the L/S of 70:1.
(2) Analysis of dissolved residues showed that the dissolution process of kaolinite in alkali solution was carried out by peeling damage on crystal plane and insertion damaged in interlayer synchronously. The DSP formed in this experiment was hydroxyl-sodalite with lamellar structure, which precipitated on kaolinite and hindered the dissolution of kaolinite.
(3) Because of the precipitation of DSP, the dissolution ratio of kaolinite was decreased. Under the optimum dissolution condition, the dissolution ratio of kaolinite reached 55%. The A/S of dissolved residues was increased to 8.55, while the A/S of the bauxite was only 4.97.
References
[1] ZHANG Ning-ning, NGUYEN A V, ZHOU Chang-chun. A review of the surface features and properties, surfactant adsorption and floatability of four key minerals of diasporic bauxite resources [J]. Advances in Colloid and Interface Science, 2018, 254: 56-75.
[2] BI Shi-wen, YU Hai-yan, YANG Yi-hong, ZHAO Fu-hui, YIN Zhong-lin, ZHAI Xiu-jing. Bayer process for the production of alumina [M]. Beijing: Metallurgical Industry Press, 2007. (in Chinese)
[3] LI Xiao-bin, WANG Yi-lin, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, WANG Hong-yang. Transformation of hematite in diasporic bauxite during reductive Bayer digestion and recovery of iron [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 2715-2726.
[4] PAN Xiao-lin, YU Hai-yan., TU Gan-feng, BI Shi-wen. Effects of precipitation activity of desilication products (DSPs) on stability of sodium aluminate solution [J]. Hydrometallurgy, 2016, 165: 261-269.
[5] JIANG Tao, PAN Xiao-lin, WU Yan, YU Hai-yan, TU Gan-feng. Mineral transition of desilication products precipitated in synthetic sodium aluminate solution under atmospheric pressure [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 367-375.
[6] BARNES M C, JONAS A M, GERSON A R. The kinetics of desilication of synthetic spent Bayer liquor seeded with cancrinite and cancrinite/sodalite mixed-phase crystals [J]. Journal of Crystal Growth, 1999, 200: 251-264.
[7] GIBSONA B, WONYENA D G, CHELGANI S C. A review of pretreatment of diasporic bauxite ores by flotation separation [J]. Minerals Engineering, 2017, 114: 64-73.
[8] MASSOLA C P, CHAVES A P, LIMA J B, ANDRADE C F. Separation of silica from bauxite via froth flotation [J]. Minerals Engineering, 2009, 22: 315-318.
[9] GRAFE M, POWER G, KLAUBER C. Bauxite residue issues III: Alkalinity and associated chemistry [J]. Hydrometallurgy, 2011, 108: 60-79.
[10] LI Xiao-bin, WANG Hong-yang, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, WANG Yi-lin. Efficient separation of alumina and silica in reduction-roasted kaolin by alkali leaching[J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 416-423.
[11] LI Xin-hua, GU Song-qing, YIN Zhong-lin, WU Guo-bao, ZHAI Yu-chun. Regulating the digestion of high silica bauxite with calcium ferrite addition [J]. Hydrometallurgy, 2010, 104(2): 313-316.
[12] LI Xiao-bin, WANG Hong-yang, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, WANG Yi-lin. Reaction behavior of kaolinite with ferric oxide during reduction roasting[J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 186-193.
[13] PAN Xiao-lin, YU Hai-yan, DONG Kai-wei, TU Gan-feng, BI Shi-wen. Pre-desilication and digestion of gibbsitic bauxite with lime in sodium aluminate liquor [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(11): 973-977.
[14] PAN Xiao-lin, YU Hai-yan, TU Gan-feng. Reduction of alkalinity in bauxite residue during Bayer digestion in high-ferrite diasporic bauxite [J]. Hydrometallurgy, 2015, 151: 98-106.
[15] JIANG Tao, LI Guang-zhu, QIU Guan-zhou, FAN Xiao-hui, HUANG Zhu-cheng. Thermal activation and alkali dissolution of silicon from illite [J]. Applied Clay Science, 2008, 40(1-4): 81-89.
[16] PENG Hong, VAUGHAN J, VOGRIN J. The effect of thermal activation of kaolinite on its dissolution and reprecipitation as zeolites in alkaline aluminate solution [J]. Applied Clay Science, 2018, 157: 189-197.
[17] ZHONG Hong, LIU Guang-yi, XIA Liu-yin, LU Yi-ping, HU Yue-hua, ZHAO Sheng-gui, YU Xin-yang. Flotation separation of diaspore from kaolinite, pyrophyllite and illite using three cationic collectors [J]. Minerals Engineering, 2008, 21(12-14): 1055-1061.
[18] LIU Gui-hua, LI Xiao-bin, PENG Zhi-hong, ZHOU Qiu-sheng. Stability of calcium silicate in basic solution [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(5): 1235-1238.
[19] BROWN I W M, MACKENZIE K J D, BOWDEN M E, MEINHOLD R H. Outstanding problems in the kaolinite–mullite reaction sequence by 29Si and 27Al solid-state nuclear magnetic resonance II: High-temperature transformations of metakaolinite [J]. Journal of the American Ceramic Society, 1985, 68: 298-301.
[20] CHAKRABORTY A K, GHOSH D K. Re-examination of kaolinite- to-mullite reaction series [J]. Journal of the American Ceramic Society, 1978, 61: 170-173.
[21] SANTOS H D, CAMPOS T W, SANTOS P D, KIYOHARA P K. Thermal phase sequences in gibbsite/kaolinite clay: Electron microscopy studies [J]. Ceramics International, 2005, 31: 1077-1084.
[22] SMITH P. The processing of high silica bauxites-Review of existing and potential processes [J]. Hydrometallurgy, 2009, 98: 162-176.
[23] XU Bing-an, SMITH P, DESILVA L. The Bayer digestion behavior of transition aluminas formed from roasted gibbsite [J]. International Journal of Mineral Processing, 2013, 122: 22-28.
[24] ZHOU Qiu-sheng, LI Chuang, LI Xiao-bin, PENG Zhi-hong, LIU Gui-hua, QI Tian-gui. Reaction behavior of ferric oxide in system Fe2O3-SiO2-Al2O3 during reductive sintering process [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 842-848.
[25] XU Bing-an, SMITH P. Dehydration kinetics of boehmite in the temperature range 723-873 K [J]. Thermochimica Acta, 2012, 531: 46-53.
[26] XU Bing-an, WINGATE C, SMITH P. The effect of surface area on the modelling of quartz dissolution under conditions relevant to the Bayer process [J]. Hydrometallurgy, 2009, 98: 108-115.
[27] LI Guang-hui, JIANG Tao, QIU Guan-zhou, FAN Xiao-hui, JIANG Hao. Technology and mechanism of desilication from roasted diasporic bauxite in atmosphere [J]. Transactions of Nonferrous Metals Society of China, 2002, 12: 132-136.
[28] PENG Hong, SENEVIRATNE D, VAUGHAN J. Role of the amorphous phase during sodium Aluminosilicate precipitation [J]. Industrial and Engineering Chemistry Research, 2018, 57: 1408-1416.
[29] BISH D L. Rietveld refinement of non-hydrogen atomic positions in kaolinite [J]. Clays and Clay Minerals, 1989, 37: 289-296.
[30] WANG Hao, FENG Qi-ming, LIU Kun. The dissolution behavior and mechanism of kaolinite in alkali-acid leaching process [J]. Applied Clay Science, 2016, 132-133: 273-280.
[31] MA Jia-yu, LI Zhi-bao, XIAO Qing-gui. A new process for Al2O3 production from low-grade diasporic bauxite based on reactive silica dissolution and stabilization in NaOH-NaAl(OH)4 media [J]. AIChE Journal, 2012, 58: 2180-2191.
[32] YANG Hui-bin, PAN Xiao-lin, YU Hai-yan, TU Gan-feng, SUN Jun-min. Dissolution kinetics and mechanism of gibbsitic bauxite and pure gibbsite in sodium hydroxide solution under atmospheric pressure [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(2): 4151-4159.
[33] ROACH G I D, WHITE A J. Dissolution kinetics of kaolin in caustic liquors [C]//Essential Readings in Light Metals. Springer eBook: Springer Nature, 2016: 240-246
[34] BARNES M C, ADDAI J, GERSON A R. A methodology for quantifying sodalite and cancrinite phase mixtures and the kinetics of the sodalite to cancrinite phase transformation [J]. Microporous and Mesoporous Materials, 1999, 31: 303-319.
[35] YAO Zhi-tong, LI Hai-yan, XIA Mei-sheng, YE Ying, ZHANG Lu. Hydrothermal synthesis of sodalite from coal fly ash and its property characterization [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(2): 366-371. (in Chinese).
一水硬铝石型铝土矿中高岭石的常压碱溶动力学与溶出脱除机理
吴 艳,潘晓林,韩月娇,于海燕
东北大学 冶金学院,沈阳 110819
摘 要:提出一种一水硬铝石型铝土矿中高岭石的常压化学预脱硅新工艺。通过化学分析、XRD和SEM对溶出过程动力学及溶出机理进行研究。溶出动力学结果表明,高岭石与一水硬铝石虽嵌布共生,但并无包覆现象。因此,在100 °C下,高岭石溶出90 min的溶出率可接近100%。溶出动力学方程为1-(1-α)1/3=7.88×106exp[-64434/ (RT)]t。低液固比(L/S=10:1)条件下,高岭石的溶出率降低到55%。这是由于形成层状羟基方钠石(OH—SOD),并沉淀在高岭石的表面,阻碍高岭石进一步被溶出。在最优条件下,可以将溶出渣的铝硅比提高到8.55,而原矿中铝硅比仅为4.97。
关键词:拜耳法;高岭石;预脱硅;溶出动力学;方钠石
(Edited by Xiang-qun LI)
Foundation item: Project (2018YFC1901903) supported by the National Key Research and Development Program of China; Projects (51774079, 51674075) supported by the National Natural Science Foundation of China; Project (N182508026) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Xiao-lin PAN; Tel: +86-24-83686460; E-mail: panxl@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(19)65169-1
Abstract: A new chemical pre-desilication process of kaolinite in diasporic bauxite in alkali solution at atmospheric pressure was proposed. The dissolution kinetics and mechanism were studied by chemical analysis, XRD and SEM. The kinetic results of dissolution process show that the kaolinite is symbiotic with diaspore but without cladding. The dissolution ratio of kaolinite is close to 100% at 100 °C for 90 min. The dissolution kinetic equation is 1-(1-α)1/3=7.88×106exp[-64434/(RT)]t. With the low L/S (L/S= 10:1), the dissolution ratio of kaolinite decreases to 55%. This is due to the formation of lamellar hydroxyl—sodalite (OH—SOD) which is deposited on the surface of kaolinite and hinders the further dissolution of kaolinite. Under the optimum conditions, the A/S (mass ratio of Al2O3 to SiO2) of dissolved residues is increased to 8.55, while the A/S of the bauxite is only 4.97.


