
Characteristics and properties of surface coated nano-TiO2
GAO Jia-cheng(高家诚), ZOU Jian(邹 健), TAN Xiao-wei(谭小伟), WANG Yong(王 勇)
College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China
Received 10 February 2006; accepted 18 July 2006
Abstract:
Nano-TiO2 was coated with A12O3, SiO2 and silane coupling agent by chemical liquid deposition. The coating was characterized by Raman spectroscopy, XRD, TEM and FT-IR. The coating content and anti-ultraviolet capacity of nano-TiO2 were measured by XRF and UV-vis spectrometer. The results show that dense coatings containing 5% Al2O3 or SiO2 can be obtained by mixing slurry at pH 10, adding coating reagent and neutralization reagent into the slurry for 60 min at 85-95 ℃, and finally aging for 120 min. Noncrystal SiO2 was coated on the surface of nano-TiO2 to form silica gel polymer with a Ti—O—Si bond, while aluminum compound exists in the form of AlOOH and part Al(OH)3. The integrated dense film can shield photocatalysis effectively. The inorganic coating film can increase the wettability for xylene and stability in water. The surface modification of nano-TiO2 will not impair its ability for anti-ultraviolet radiation, and more short band ultraviolet radiation can be absorbed. In addition, the optimal coating amount of silane coupling agent should be less than 3% and the best wettability for xylene can be reached when the amount is 1.2%.
Key words:
nano-TiO2; surface modification; coating; weatherability; dispersing;
1 Introduction
Nano-TiO2, as a new inorganic material, has many excellent properties,such as nontoxic, stable in chemistry and high efficient photocatalytic effect[1,2]. As fine particles, it is easy to agglomerate in practical use, especially difficult to disperse in organic solvent[3]. And because of its strong photocatalytic effect, especially under ultraviolet radiation it will lead painting to powder and lower weatherability[4]. Surface modification of nano-TiO2 material has been reported from a lot of researches. They focused mainly on the coating process and the development of different coating materials while a little on surface coating characteristics and properties; also the mechanism for film coating was not discussed in detail. The effective characterization and better properties after surface modification are key problem also. In this work, nano-TiO2 is coated by inorganic oxide to close its high activity, improve the weatherability and keep high lightness[5], and then is modified by organics to disperse in organic solvent stably[6].
2 Experimental
The experimental materials included 6% nano-rutile slurry, sodium silicate coating agent (100 g/L), partial sodium aluminate (100 g/L), aluminum sulphate (100 g/L), hydrophile silane coupling agent, hexad partial sodium phosphate (100 g/L) dispersant, xylene and self-made desalt water. The regulators for pH were sulphuric acid, hydrochloric acid, sodium hydroxide with different concentrations.
Slurry was adjusted to suitable pH value about 8-11 and mixed efficiently at room temperature. Then it was neutralized to constant pH value about 7. The coating temperature is 85-95 ℃. After sampling at different time, vacuum filtrating, cleaning and drying were done. Excessive silane coupling agent was added at different time for organic coating, to fix on the surface of nano-TiO2 uniformly. The coated powders were washed several times and then roasted at 105 ℃ for 24 h.
The uncoated and coated titania powders were analyzed quantitatively by X-ray fluorescence spectrum. The organic coating sample was calcined in mufflefurnace at 850 ℃ to measure the mass loss. The coating amount can be obtained from it. Phase analysis was carried out on Japan D/MAX-1200 full-automation X-ray diffraction and infrared radiation spectrophoto- meter. Various slurry materials and nano-TiO2 samples before and after coating were measured by Raman analyzer at Peking University. The dispersing stability and wetting angle of each sample were also measured.
3 Results and discussion
3.1 Coating layer characteristics
The XRD spectra of uncoated and coated nano-TiO2 are shown in Fig.1. It can be seen that the uncoated sample is in rutile state. The average grain size of particle is 57 nm, calculated by Scherrer formula[7]. The XRD spectra of uncoated and coated nano-TiO2 are similar, and the coated samples do not contain the diffraction peaks of other oxide crystal. Fig.2 shows the XRD spectra of coated specimen calcined at 1 300 ℃ for 1 h. Apart from rutile TiO2, there exist Al2O3 in Fig.2(a) and SiO2 in Fig.2(b). There are both Al2O3 and SiO2 phases in specimen coated with Al2O3 and SiO2 (Fig.2(c)). These results indicate that the coatings are noncrystal before calcining, while become crystal A12O3 and SiO2 after being calcined at 1 300 ℃.
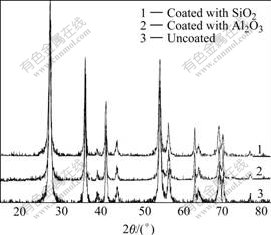
Fig.1 XRD spectra of uncoated and coated nano-TiO2
In order to investigate the surface modification effect on nano-TiO2, the film morphology of samples before and after treatment was observed by TEM. Fig.3(a) shows the TEM morphology of unmodified nano-TiO2, Figs.3(b)-(d) show the TEM pictures of nano-TiO2 particles coated with Al2O3 in acidic condition, with Al2O3 in alkaline condition and coated with Al2O3 and SiO2, respectively. Compared with Figs.3(a) and (b), the dispersing degree of samples shown in Figs.3(c) and (d) is greatly improved. And there are no spherical alumina particles in Figs.3(b) and (c).
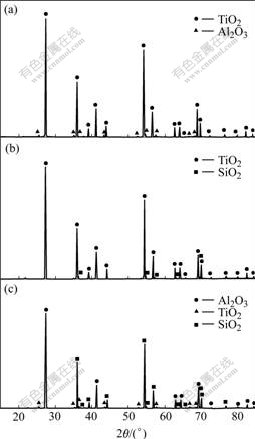
Fig.2 XRD spectra of coated specimen calcined at 1 300 ℃: (a) Coated with Al2O3; (b) Coated with SiO2; (c) Coated with Al2O3/SiO2
The IR spectra of coated specimens are shown in Fig.4. The absorption peak at 1 096 cm-1 is caused by flex vibration of Si—OH group, the one at 891 cm-1 by Si—O—Si and Ti—O—Si bond and the one at 3 416 cm-l by flex vibration of —OH polymerization, which is in superposition with the absorption peak of crystal water in Fig.4(a). Combined with XRD analysis, it is shown that the coating film of nano-TiO2 coated with SiO2 is amorphous silicon-oxygen polymerization substance. It is also shown that the coating film of nano-TiO2 coated with Al2O3 exists in the form of AlOOH and amorphous Al(OH)3 (Fig.4(b)). The IR spectrum of the specimen coated with Al2O3 and then modified with organic substance is shown in Fig.4(c). Compared Fig.4(b) with Fig.4(c), it is shown that the —OH flex vibration peaks of AlOOH at 2 986 cm-1, 2 934 cm-l , 2 852 cm-1 are reduced to two peaks (a strong and a weak) after organic modification, and the relative intensity is strengthened because of CH2— and CH3—groups. The shape also varies at 1 388 cm-1 and a new absorption peak occurs at 1 265 cm-1 after modification. The intensity of absorp- tion peak at l 096 cm-1 and l 055 cm-1 is strengthened after modification, especial for that at 886 cm-1. The absorption peak at 800 cm-1 occurs after organic modification, which is caused by the vibration absorption of Si—O bond in silane coupling agent. Therefore nano-TiO2 coated with silane coupling agent has organic silane coatings.
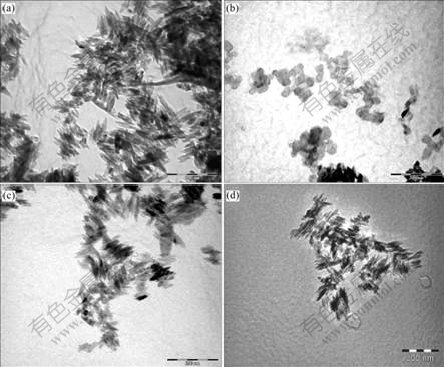
Fig.3 TEM morphologies of samples coated under different conditions: (a) Uncoated; (b) Coated with Al2O3 in acidic condition; (c) Coated with Al2O3 in alkaline condition; (d) Complexly coated with Al2O3 and SiO2
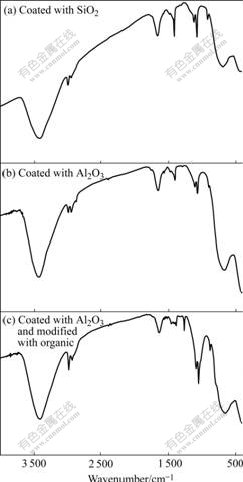
Fig.4 IR spectra of coated Al2O3 specimen
Fig.5 shows Raman spectra of nano-TiO2 specimens coated and uncoated with Al2O3. In Fig.5(a), there are two strong characteristic peaks of rutile between 250-500 cm-1. While in Fig.5(b), two characteristic peaks of alumina stronger than rutile peak in intensity exist between l 000-1 250 cm-1. This indicates that the surface of nano-TiO2 is coated with alumina.
The relative content of oxides on the nano-TiO2 power is shown in Table 1. The samples of series 1 mean for complex coating film coated first with SiO2 and then with Al2O3 in proportion of l:2, l:l, 3:2. It can be seen from Table 1 that the result is similar to the charge quantity. The series 2 represents samples coated with SiO2, where sample 2-1 is held for 30 min, with less half amount of coating material than sample 2-2, since coating agent can not deposit on nano-TiO2 surface completely in short holding time. The series 3 means samples coated with Al2O3, where sample 3-1 is coated in acid condition and sample 3-2 in alkali conditions, both with 5% charge.
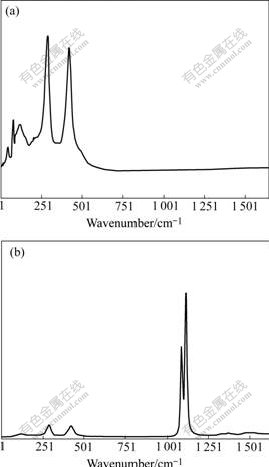
Fig.5 Raman spectra of nano-TiO2 powder specimen uncoated(a) and coated with alumina(b)
Table 1 Result of XRF analysis on partial specimen
The excessive quantity of 50% polymer is used and the remainder is removed with high speed centrifugal machine in the trial. Therefore, it can be regarded that coating agent absorbs tightly on the surface. The coating rates in Table 2 present that coating agents can not be removed by centrifugal process[8].
3.2 Properties of coated nano-TiO2
The ultraviolet radiation transmittivity of sample is shown in Fig.6, where samples 1-5 represent the unmodified, SiO2 and Al2O3 complexly coated, SiO2 coated, Al2O3 coated, and A12O3 and SiO2 coated respectively. The ultraviolet radiation transmittivity of all samples at 260-400 nm is nearly zero, while at 200-260 nm it is different, especial for sample l the transmittivity is 14% and for others the transmittivity is 7%. In fact only the middle-length wave band of ultraviolet radiation from sun to earth (290- 400 nm) is harmful to human. So the wave band of ultraviolet radiation can be well shielded and the requirement of anti-ultraviolet radiation can be met by use of coated samples.
Table 2 Mass loss of specimen coated with polymer after being calcined at 850 ℃
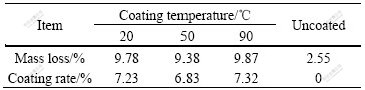
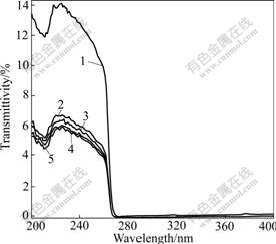
Fig.6 Ultraviolet transmittivity of specimens
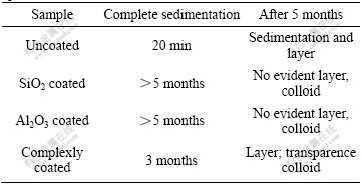
The settlement experiment result is shown in Table 3. For uncoated samples, the complete sedimentation only need 20 min, which means poor dispersing stability, and for coated samples there is a little sedimentation in 5 months, which means good dispersing stability in distilled water. For Al2O3 and SiO2 complexly coated samples, however, the slurry concentration is gradually increased from the top to bottom. In fact there is no evident boundary and the liquid in upper layer is colloid of milkiness transparence.
Table 3 Result of sedimentation experiment for various specimens
The dispersing stability of modified nano-TiO2 in distilled water is improved greatly. According to DLVO theory, the stability of colloid is determined by the relative size of repulsion potential energy and attraction potential energy of colloid particle[9]. The attraction potential energy bears a relation with surface property of particle and repulsion potential energy with electrical potential of nano-TiO2 surface. At different pH values, the electrical potential is decided by particle surface structure, for example, the equipotential (=0) of hydrated A12O3 responds to pH 12, hydrated SiO2 to pH 2, nano-TiO2 to pH 6 and general rutile titania to pH 3.6[10].
The surface structure of particle may be altered by surface coating, for example, the surface of normal rutile TiO2 coated with 0.5% hydrated SiO2 is consistent with hydrated SiO2[11]. The surface structure of samples coated with Al2O3 is similar to hydrated Al2O3 and the equipotential is at pH 12, while the pH value of distilled water is close to neutrality far from equipotential, so the higher relative electrical potential and the larger repulsion force is, the better the dispersing stability is. After coated with SiO2, the equipotential of hydrated SiO2 is at pH 2, far from pH 7, so there is good dispersing stability also. And the pH value of uncoated nano-TiO2 is 6-7, which is similar to the pH value of distilled water. As a result, the repulsion force between particles is small and large attraction force lead particles to agglomerate together.
Through varying the proportion of A12O3/SiO2 on surface coating particle, different electric properties are obtained, such as, positive electricity at A12O3/SiO2>1 and negative at Al2O3/SiO2<1. The final slurry delaminates quickly at A12O3/SiO2 about 1, but it is difficult to delaminate at A12O3/SiO2 about 0.47 or 1.5 in this trial.
The h2—t plots of water and TiO2 coated and uncoated sample are shown in Fig.7. Based on Washbum formula, that is h2=(γcosθ?σ?t)/2ηL (defining R=γcosθ), factors such as R1=7.29×10-4; R2=2.45×10-4; R3= 6.86×10-4; R4=3.43×10-4; i.e. R1>R3>R4>R2, γ constant; θ2>θ4>θ3>θ1 are obtained. So the hydrophilicity of nano-TiO2 will be altered through surface modification.
The h2—t plots of xylene and TiO2 inorganic- organic coated and uncoated are shown in Fig.8. It can be seen that the sample coated with SiO2 has the maximum slope, which corresponds to the smallest R and θ, i.e. best lipophilicity. Coating with A12O3 and complex coating samples that modified by organic make the lipophilicity of nano-TiO2 reduce, among them the lowest lipophilicity is for samples coated with A12O3 and modified by organic.
The R curves of the amount of organic modifiers are shown in Fig.9. It is shown that first, R is evidently increased with coating amount increasing and the largest R occurs at 1.2% coating amount. Then it decreases with coating amount increasing and keeps constant at 3% coating amount.
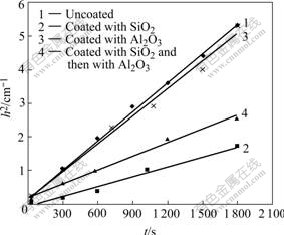
Fig.7 h2—t plots of water and TiO2 samples
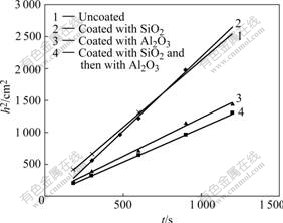
Fig.8 h2—t plots of xylene and TiO2 modified with silane coupling agent
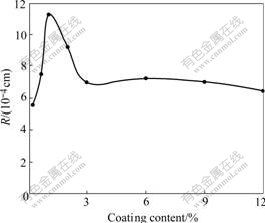
Fig.9 Effect of amount of silane coupling agent on nano-TiO2 wetting capacity
TiO2 has good hydrophilicity since hydroxyl formed through water polarization. The hydrophilicity of Al2O3/ SiO2 is poor because of its weak polarization. Therefore, it affects the paint absorption of modified nano-TiO2 surface. Obviously the kind and amount of coating agent will affect the hydrophilicity of nano-TiO2. Large polarization hydroxyl is reduced by coated hydrated SiO2 with weak polarization. In Fig.9, the wettability change with coating content has an extremum. The wettability is mainly determined by surface radicle, similar with inorganic surface modification. Along with organic content increment, hydrophilia —OH is gradually replaced by paint absorption function group in silane coupling agent. OH disappears at 1.2% coating content, having the best paint absorption. In fact, organic coating on inorganic surface is really a chemical absorption that forms organic film. It can be divided into two stages[12], i.e. single molecule absorption for good hydrophilicity and surface colloid for opposite. Single molecule absorption occurs when the content of silane coupling agent in coating is below 1.2% and complete single molecule film is formed at 1.2% content, i.e. nano-TiO2 coated completely with silane coupling agent, as the best silane coupling agent. However it is opposite paint absorption with colloid increment, by which single molecule layer is replaced at 3%. This is a reason why stable paint absorption is at over 3% silane coupling agent.
3.3 Influence factors of coating
The pH value will affect the dispersion of slurry and the coating process. In this trial, nucleus coating is suitable for acidity and film coating for alkali conditions at same A12O3 content. There is the better coating film at pH 8-10 for samples coated with SiO2.
Mixing slurry sufficiently can get the high dispersion, supersaturating and uniform pH of coating material, so as to form a full uniformity film. For organic film, sufficient stirring is needed to disperse particles and form a fully uniform organic film on nano-TiO2.
Dispersing time means stirring time before coating. Short time stirring will induce insufficient dispersion for slurry, which leads more particles group to be coated together without nano-particle. If it is too short, it will make the pH value non-uniform. Neutralizing time should be long enough. If it is too short, it will make coating film not coat on nano-TiO2 surface but increase the supersaturation of slurry, which finally forms nucleus film, such as 1 h neutralizing for coated SiO2. Sufficient holding time will make coating material completely coat on the surface of particle and form a dense film, for example, a dense film has been gained by holding coated SiO2 sample for 3 h.
The coating temperature will greatly affect the property of slurry. If it is too low, the diffusion rate of coating material is relatively slow, which is easy to form an abnormal coating. If the temperature is too high, such as above boiling point, water in slurry will easily vaporize and the concentration greatly varies, which is difficult to form the film. General coating temperature should not be lower than 60 ℃, only organic coating forms covalent bond when heating for dehydration.
The pH and concentration may be controlled in a small range by using the process of first dripping coating agent and then adding neutralizing agent. Such as in coated SiO2 process it will get dense film to keep over pH 9. Contrarily it will make pH or coating agent non-uniform at local part by using the process of adding coating material and neutralizing agent at the same time. Concentration of neutralizing agent should be about 1%. If it is too high, it will make the pH or coating agent locally non-uniform and obtain a poor coating.
4 Conclusions
1) A dense film containing 5% SiO2 or A12O3 can be obtained by sufficiently stirring the slurry at pH 10, neutralizing for 60 min at 85-95 ℃ and aging for 120 min.
2) The uniform film on nano-TiO2 coated with SiO2 has a microstructure of internal Si—O—Si bond, Si—OH group on the surface and Ti—O—Si bond combining with nano-TiO2. For Al2O3 coating, it is AlOOH and Al(OH)3.
3) The growth of karyotheca is superior to new nucleus formation. The longer the aging time is, the denser the coating is. Nucleus coating is easily obtained in acid slurry with insufficient mixing. Complete dense coating film has good shielding ability for nano -TiO2.
4) When modifying with silane coupling agent, the surface active —OH on nano-TiO2 is a key. The content of silane coupling agent can reach up to 7%. The best wettability is at 1.2% silane coupling agent and the invariableness wettability is at 3%.
5) The dispersing stability and wettability of nano-TiO2 are improved efficiently through the surface modification. The surface coating does not reduce the absorption of ultraviolet radiation for nano-TiO2.
References[1] ZHANG Ru-bin, GAO Lian. Effect of peptization on phase transformation of TiO2 nanoparticles [J]. Mater Res Bull, 2001, 36: 1957-1965.
[2] YANG Juan, MEI Sen, FERREIRA J M F. Hydrothemal synthesis of nanosized titania powders: Influence of tetraalkyl ammonium hydroxides on particle characteristic [J]. J Am Ceram Soc, 2001, 84(8): 1696-1706.
[3] NAVIO A, CILON G. Iron-doped titania semiconductor powders prepared by a sol-gel method (Part I): Synthesis and characterization [J]. Appl Catal A: General, 1999, 177(1): 111-120.
[4] WU Jian-chun, HUANG Wan-xia, ZHENG Hong-ping, CAO Jian-jun. Research on weatherability of nano-TiO2 modifying exterior latex paint [J]. Iron Steel Vanadium Titanium, 2003, 24(4): 55-58. (in Chinese)
[5] PAUL S. Surface Coating: Science and Technology [M]. 2nd ed. Chichester: Wiley Publication, 1996: 354.
[6] MANORAMA S V, MDDHUSUDAN REDDY K, GOPAL REDDY C V, et al. Photostabization of dye on anatase titania nanoparticles by polymer capping [J]. Journal of Physics and Chemistry of Solids, 2002, 63: 135-143.
[7] LIU X, WANG X, LU L. Study of nanocrystalline TiO2 prepared with raw and modified gelatin dispersants [J]. J Appl Polym Sci, 1999, 141: 60-69.
[8] LIN Yu-lan, WANG Ting-jie, QIN Cao, YANG Jun. Organic modification on the sub-micron particles of TiO2 pre-coated with SiO2 and Al2O3 [J]. Chemical Journal of Chinese Universities, 2001, 22(1): 104-107. (in Chinese)
[9] LIU Yang-Qiao, GAO Lian. Investigation on properties of nano-sized 3Y-TZP aqueous suspensions [J]. Chemical Journal of Chinese Universities, 2002, 23(11): 2155-2158. (in Chinese)
[10] CUI Ai-li, WANG Ting-jie, HE Hong, JIN Yong. Dispersion behavior of ultrafine titanium dioxide particles in aqueous solution [J]. The Chinese Journal of Process Engineering, 2001, 1(1): 99-101. (in Chinese)
[11] QIN Cao, WANG Ting-Jie, JIN Yong. The coating process of nano-scale hydrous silica film on TiO2 particles by chemical deposition in aqueous solution [J]. Acta Phys Chim Sin, 2002, 18(10): 884-889.
[12] LI Xing-wei, CHEN Wei, BIAN Chao-qing, HE Jin-bo, XU Ning, XUE Gi. Surface modification of TiO2 nanoparticles by polyaniline [J]. Applied Surface Science, 2003, 217: 16-22.
(Edited by YUAN Sai-qian)
Foundation item: Project(2002126) supported by the Chongqing Science Committee, China
Corresponding author: GAO Jia-cheng; Tel: +86-23-65102821; E-mail: gaojch@cqu.edu.cn


