
Electrochemical corrosion behavior of Cu-40Ni-20Cr alloys with
different grain sizes in solutions containing chloride ions
CAO Zhong-qiu(曹中秋), BIAN Jing(边 静), XUE Rong(薛 荣), LIU Wei-hua(刘伟华)
Department of Chemistry, Shenyang Normal University, Shenyang 110034, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The electrochemical corrosion behavior of the two Cu-40Ni-20Cr alloys prepared by conventional casting(CA) and mechanical alloying(MA) with the different grain sizes was studied by using open-circuit potential(OCP), potentiodynamic polarization and electrochemical impedance spectroscopy(EIS) methods in solutions containing chloride ions. The results show that the free corrosion potentials of the two alloys move towards negative values, corrosion currents increase and therefore corrosion rates become faster with the increase of chloride ion concentrations. EIS plots of CACu-40Ni-20Cr alloy are composed of single capacitive loop, while EIS plots of MACu-40Ni-20Cr alloy are composed of double capacitive loops in solution containing lower chloride ion concentrations. EIS plots of the two alloys have Warburg impedance with the increase of chloride ion concentrations. Corrosion rates of MACu-40Ni-20Cr alloy become faster than those of CACu-40Ni-20Cr alloy obviously in solutions containing the same chloride ion concentrations
Key words:
Cu-Ni-Cr alloy; grain size; electrochemical corrosion; microstructure;
1 Introduction
Usually, the corrosion behavior of nanocrystalline materials is different from that of corresponding coarse grained materials due to a large reduction of grain size[1-3]. Thus, whether nanocrystalline materials have good corrosion resistances or not have attracted extensive attention from some material scientists. For example, WANG and LI[4] found that corrosion resistances of nanocrystalline surface of 304 stainless steel prepared by sandblasting and annealing process were improved in 3.5% NaCl solution because of faster diffusion of passive element in the increased grain boundaries of nanocrystalline materials which favored a rapid formation of the protective film. LU et al[5] reported the dissolution rate of the sputtered nanocrystalline Cu-20Zr films was lower than that of the cast alloy because of the electrochemical activity of Zr in the different phases of the samples. BARBUCCI et al[6] found that nanocrystalline Cu90Ni10 alloys had faster corrosion rates than corresponding coarse grained alloys. CAO et al[7-8] also compared the electrochemical corrosion behavior of nanocrystalline Cu-40Ni alloys with the coarse grained alloys in solutions containing Cl- or H+ and found a decrease of corrosion resistances in the nanocrystalline alloys because of the increased number of reactive atoms on alloy surface. However, no determination of electrochemical corrosion mechanism of nanocrystalline alloys is available at present, which limits the practical applications of nanocrystalline alloys [6-8].
Mechanical alloying as a kind of method of preparing nanocrystalline powders has been widely used in powder metallurgy. It has special importance for these alloy systems in which very small mutual solubility of element occurs either in solid state or in liquid phase such as W-Ag, W-Cu, Ag-Ni and Cu-Cr alloys, which are useful contact materials in electric industry but they are difficult to prepare by conventional technique[9-11]. Hot pressing is an effective densification method for preparing bulk nanocrystalline materials[12-13] such as Ag-Ni and Cu-Cr.
Cu-Ni alloys have been used extensively as pipelines,structural materials and ship hulls in marine environments because they have corrosion resistance, mechanical ductility, excellent electrical and thermal conductivity, and excellent anti-fouling properties[14]. Additions of Cr to Cu-Ni alloys have been expected to improve the corrosion resistances since Cr is a noble element.
The aim of this work is to examine the electrochemical corrosion behavior of bulk nano- crystalline Cu-40Ni-20Cr alloy prepared by mechanical alloying(MA) and hot pressing process when exposed to solutions containing chloride ions and compare their electrochemical corrosion behavior with coarse grained Cu-40Ni-20Cr alloy prepared by conventional casting (CA) technique in the favor of developing a better understanding on the electrochemical corrosion mechanism of nanocrystalline alloys.
2 Experimental
2.1 Alloy preparation
A Cu-Ni-Cr alloy containing (molar fraction, the same below) 40% Cu, 40% Ni and 20% Cr (denoted here as CACu-40Ni-20Cr) was prepared by conventional casting appropriate mixtures of the pure components (99.99 %) under a Ti-gettered argon atmosphere using non-consumable tungsten electrodes. The alloy ingot was subsequently annealed in vacuum at 800 ℃ for 54 h to remove residual mechanical stresses and to achieve a better equilibration of the alloy phases with about 120 μm in grain size. The actual average composition of this alloy was 39.3% Cu, 41.0% Ni and 19.7% Cr according to analysis by scanning electron microscopy and energy-dispersive X-ray microanalysis(SEM/EDX).
A Cu-Ni-Cr alloy containing 40% Cu, 40% Ni and 20% Cr (denoted here as MACu-40Ni-20Cr) was prepared by mechanical alloying (MA) and hot pressing process. Elemental Cu, Ni and Cr powders (purity >99.9%) with particle size about 100 μm were blended with appropriate amounts of the powders of the three metals. Mechanical alloying was carried out in a QM-1SP planetary miller. Three powders were first stir-mixed in a stainless steel vial with hardened GCr15 steel ball. The mass ratio of ball to powder was about 10?1. The vial with powders was evacuated to about 10 Pa and then filled with pure argon. During ball milling a relay was used to control intermediate stops of 15 min every 1 h to avoid excessive heat effect. The whole powders were prepared by mechanical alloying for 60 h in order to obtain alloying on atomic level. Nearly fully dense sample was prepared by hot pressing at the temperature of 800 ℃ for 10 min and the pressure of 20 MPa in vacuum of about 0.06 Pa using a graphite die with inner diameter of 20 mm. During hot pressing all signals including the temperature, its displacement and deformation can be attributed to a computer, from which the deformation and densification curve can be obtained[12]. Finally, the resulting ingot was annealed for 1 h at 800 ℃ to stabilize the grain structure and relieve mechanical stresses induced by hot pressing. Measurements of the average grain size using the profile of the X-ray diffraction peaks gave a value of about 8 nm after ball milling, which increased up to 25 nm after hot pressing and to 33 nm after annealing.
Flat specimens were cut from the alloy ingots by a diamond-wheel saw, which was embedded in epoxy resin and area exposed was 1 cm2. The specimen surface was mechanically polished emery up 1000 grit to ensure the same surface roughness, followed by washing with acetone and di-distilled water.
2.2 Experimental method
All electrochemical measurements were carried out in 0.05 mol/L Na2SO4 neutral solutions used as base and different chloride ion concentrations such as 0.02, 0.05, 0.10 and 0.50 mol/L were added. The solutions used during the experimental work were prepared with bi-distilled water and analytical grade reagents. Open circuit potential(OCP) and potentiodynamic polarization curves were measured by PARM273A, advanced electrochemical system of Princeton applied research made in EG&G Company(USA). Electrochemical parameters as corrosion potential(Ecorr), corrosion current (Icorr), anodic and cathodic Tafel slopes (βa, βc) were fitted by Corrview software. Polarization rang was from -0.25 to 0.80 V versus OCP. The scanning rate was 0.5 mV/s. Saturated calomel electrode(SCE) was employed as the reference electrode, platinum electrode as the counter electrode and the specimens as working electrode.
Electrochemical impedance spectroscopy(EIS) was measured by M5210, advanced electrochemical system of Princeton applied research made in EG&G Company (USA). Frequency range was from 105 to 10-2 Hz. EIS analysis and circuit fitting were carried out by Zview2 software.
3 Results and discussion
3.1 Electrochemical corrosion behavior of CACu-40 Ni-20Cr
3.1.1 Open circuit potentials and potentiodynamic polarization curves
The curves of open circuit potentials of CACu- 40Ni-20Cr alloy in solutions containing different chloride ion concentrations with time are shown in Fig.1. The free corrosion potentials decrease and become stable quickly when chloride ions are added in neutral solutions.
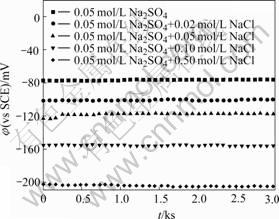
Fig.1 Curves of open circuit potentials of CACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
The free corrosion potential is -77 mV in 0.05 mol/L Na2SO4 neutral solution. With the increasing chloride ion concentrations, the free corrosion potentials are -101, -118, -156 and -205 mV, respectively. The free corrosion potentials move towards negative values in different degrees. This shows that the present alloy is very easy to be corroded with the increase of chloride ion concentrations.
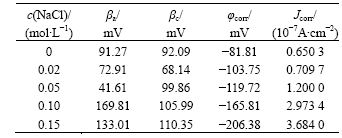
Potentiodynamic polarization curves of CACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations are shown in Fig.2. The present alloy has passivating phenomena but passivating ranges become wide and afterwards narrow with the increase of chloride ion concentrations. This shows that the present alloy may produce pitting corrosion. Electrochemical parameters fitted by Corroview software are listed in Table 1. Corrosion currents increase and corrosion rates become faster with the increase of chloride ion concentrations.
For CACu-40Ni-20Cr alloy, the main corrosion reactions are copper, nickel and chromium anodic dis-
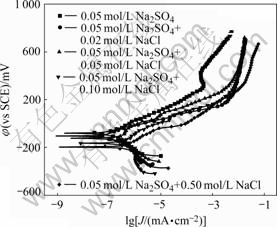
Fig.2 Polarization curves of CACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
Table 1 Electrochemical parameters of CACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
solution and oxygen cathodic reduction. The courses of anodic dissolution may be described as
Cu+Cl-→CuCl+e (1)
CuCl+Cl-→![]() (2)
(2)
Ni→Ni2++2e (3)
Cr→Cr3++3e (4)
Metal ion and chlorides were hydrolyzed into hydroxides. These processes may be described as
![]() +2H2O→Cu(OH)2↓+2HCl+e (5)
+2H2O→Cu(OH)2↓+2HCl+e (5)
Ni2++2H2O→Ni(OH)2+2H+ (6)
Cr3++3H2O→Cr(OH)3+3H+ (7)
Cathodic reduction reaction may be described as
O2+4e+2H2O→4OH- (8)
The passivating film formed on alloy surface has great influence on the electrochemical corrosion behavior. Corrosion resistances have relation to the adhesion between passivating film and matrix alloy and the ability of preventing electron and ion from getting into matrix alloy. The corrosion products Cu(OH)2, Ni(OH)2 and Cr(OH)3 are cubic lattice of p-type semiconductors in which Cu2+, Ni2+, Cr3+, or O2- can penetrate each other, therefore the ion resistances decrease. Chloride ions can exchange with O2- from corrosion products and produce some new holes in solutions containing chloride ions. These holes decrease the resistance and film protection. Thus, the corrosion rates become faster. Further more, it is more difficult to form completely passivating film on the alloy surface. Thus, the ability of ions into matrix alloy will increase.
3.1.2 EIS analysis
Electrochemical impedance spectroscopy(EIS) is a powerful tool in studying corrosion processes of alloys. EIS gives more information about the electrochemical processes taking place on the surface and is considered a good confirmation of other electrochemical techniques [15]. EIS plots of CACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations are shown in Fig.3. When CACu-40Ni-20Cr alloy was
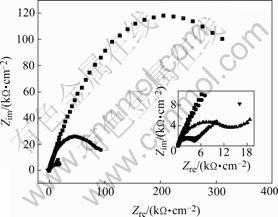
Fig.3 EIS plots of CACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations: ■ 0.05 mol/L Na2SO4; ● 0.05 mol/L Na2SO4+0.02 mol/L NaCl; ▲ 0.05 mol/L Na2SO4+0.05 mol/L NaCl; ▼0.05 mol/L Na2SO4+0.10 mol/L NaCl; ◆ 0.05 mol/L Na2SO4+0.50 mol/L NaCl
immersed in 0.05 mol/L Na2SO4 neutral solution and 0.05 mol/L Na2SO4 plus 0.02 mol/L NaCl solution, their EIS plots are composed of single capacitive loop without Warburg impedance. Thus, corrosion processes are controlled by electrochemical reactions. The equivalent circuit is shown in Fig.4. After chloride ion concentrations increase up to 0.05mol/L or more, their EIS plots consist of a capacitive loop at high frequency portion and a diffusion tail induced Warburg impedance at low frequency portion. Warburg impedance as a line with gradient of 45? at low frequency portion showed that corrosion processes were controlled by diffusion. The capacitive loop at high frequency portion is impedance of discharged process when charged particles pass double layer. The center of capacitive loops, semicircles of which are little collapsing at high frequency portion, deviate from solid axle because of dispersion effect [15]. The equivalent circuit is shown in Fig.5, where Rs is denoted as solution resistance, Rt as charge transfer resistance, Zw as Warburg impedance and CPEdl as constant phase angle element. The impedance of CPEdl could be calculated by[16]
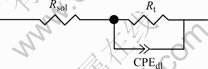
Fig.4 Equivalent circuit of CACu-40Ni-20Cr alloy in 0.05 mol/L Na2SO4 solution and 0.05 mol/L Na2SO4 plus 0.02 mol/L NaCl solution
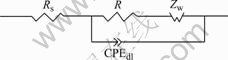
Fig.5 Equivalent circuit of CACu-40Ni-20Cr alloy in 0.05 mol/L Na2SO4 plus 0.05 mol/L or more NaCl solution
Z=Yo-1 W-n[cos(nπ/z)-jsin(nπ/z)] (9)
where Z is denoted as the impedance of CPEdl, j as imaginary part, W as angular frequency, Yo as constant and n as exponent (0≤n≤1).
Fitting results are shown in Table 2. All of charge transfer resistances decrease and therefore corrosion rates become faster with the increase of chloride ion concentrations. From Table 2, dispersion factor CPEdl-P is less than 1. It fully demonstrates that the present alloy has dispersion effect in corrosion processes.
3.2 Electrochemical corrosion behavior of MACu-40 Ni-20Cr alloy
3.2.1 Open circuit potentials and potentiodynamic polarization curves
The curves of open circuit potentials of MACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations are shown in Fig.6. The free corrosion potentials keep constant basically with the increase of time when chloride ions are added in solutions. The free corrosion potential is -101 mV in 0.05 mol/L Na2SO4 neutral solution, while the free corrosion potentials are -136, -161, -170 and -258 mV, respectively with the increase of chloride ion concentrations. The free corrosion potentials move towards negative values obviously.
Table 2 Equivalent circuit parameters of CACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
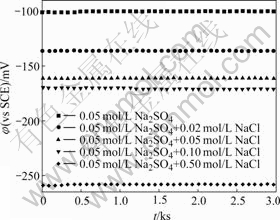
Fig.6 Curves of open circuit potentials of MACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
Potentiodynamic polarization curves of MA Cu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations are shown in Fig.7. Electrochemical parameters fitted by Corroview software are listed in Table 3. Corrosion potentials move towards negative values, corrosion currents increase and therefore corrosion rates become faster with the increase of chloride ion concentrations. When MACu-40Ni-20Cr
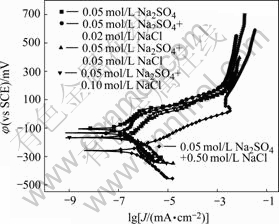
Fig.7 Polarization curves of MACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
Table 3 Electrochemical parameters of MACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
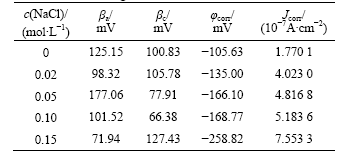
alloy was immersed in 0.05 mol/L Na2SO4 neutral solution and 0.05 mol/L Na2SO4 plus 0.02 mol/L NaCl solution, the passivating phenomena can be found in polarization curves. The passive phenomenon becomes weaker when the chloride ion concentrations increase up to 0.10 mol/L or more.
3.2.2 EIS analysis
EIS plots of MACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations are shown in Fig.8. MACu-40Ni-20Cr alloy plots are composed of double capacitive loops, a small and a big semicircle at high and low frequency portions when alloy was immersed in 0.05 mol/L neutral Na2SO4 solution and 0.05 mol/L Na2SO4 plus 0.02, 0.05, 0.10 mol/L NaCl solution. Thus, corrosion processes are controlled by electrochemical reactions. Their equivalent circuits are shown in Fig.9. After chloride ion concentrations increase up to 0.50 mol/L, its EIS plot consists of double capacitive loops and a diffusion tail induced Warburg resistance at low frequency portion. Corrosion processes are controlled by ion diffusion in passivating film. Their equivalent circuits are shown in Fig.10 where Rs is denoted as solution resistance, CPEm as electric capacity over alloy surface, Rm as resistance of electrolyte in the film, Zo as Warburg impedance. Fitting results are listed in Table 4. Charge transfer resistances decrease and therefore corrosion rates become much faster with the increase of chloride ion concentrations.
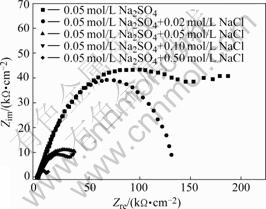
Fig.8 EIS plots of MACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
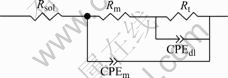
Fig.9 Equivalent circuit of MACu-40Ni-20Cr alloy in 0.05 mol/L Na2SO4 neutral solution and 0.05 mol/L Na2SO4 plus 0.02, 0.05 and 0.10mol/L NaCl solutions
Table 4 Equivalent circuit parameters of MACu-40Ni-20Cr alloy in solutions containing different chloride ion concentrations
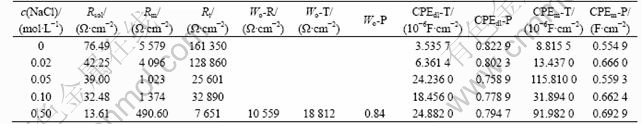
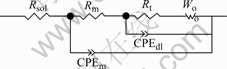
Fig.10 Equivalent circuit of MACu-40Ni-20Cr alloy in 0.05 mol/L Na2SO4 plus 0.50 mol/L NaCl solution
3.3 Effect of grain size on electrochemical corrosion behavior of Cu-40Ni-20Cr alloys
Corrosion rates of MACu-40Ni-20Cr alloy become faster than that of CACu-40Ni-20Cr alloy obviously in solution containing the same chloride ion concentration. MA Cu-40Ni-20Cr alloy has more homogeneous microstructure and is able to produce large concentrations of grain boundaries in the course of reduction in grain size by mechanically alloying. Atom in grain boundaries is arranged irregularly and its lattice distortion energy increases, grain boundaries have trend to decrease its energy automatically. When the sample was immersed in solution containing different chloride ion concentrations, there were large concentrations of grain boundaries prior others to dissolute. It could also help chloride ions to transfer freely. Thus, MA Cu-40Ni-20Cr alloy corrodes more easily than CACu-40Ni-20Cr alloy.
4 Conclusions
1) For the two Cu-40Ni-20Cr alloys, the free corrosion potentials move towards negative values and corrosion rates become faster with the increase of chloride ion concentration.
2) EIS plots of CACu-40Ni-20Cr alloy are composed of single capacitive loop, while EIS plots of MACu-40Ni-20Cr alloy are composed of double capacitive loops in solution containing lower chloride ion concentrations. Thus, corrosion processes are controlled by electrochemical reactions. EIS plots of the two present alloys have Warburg impedance with the increase of chloride ion concentrations and therefore corrosion processes are controlled by diffusion.
3) The corrosion rates of MACu-40Ni-20Cr alloy become faster than that of CACu-40Ni-20Cr alloy because the reduction in the grain size of MACu-40Ni-20Cr alloy produces large concentrations of grain boundaries.
References
[1] TJONG S C, CHEN H. Nanocrystalline materials and coatings [J]. Mater Sci Eng R, 2004, 45: 1-88.
[2] KH M S, YOUSSEF, KOCH C C, FEDKIW P S. Improved corrosion behavior of nanocrystalline zinc produced by pulse-current electrodeposition [J]. Corros Sci, 2004, 46: 51-64.
[3] LI Y, WANG F H, LIU G. Grain size effect on the electrochemical corrosion behavior of surface nanocrystallized low-carbon steel [J]. Corrosion, 2004, 60: 891–896.
[4] WANG X Y, LI D Y. Mechanical and electrochemical behavior of nanocrystalline surface of 304 stainless steel [J]. Electrochim Acta, 2002, 47: 3939-3947.
[5] LU H B, LI Y, WANG F H. Dealloying behaviour of Cu-20Zr alloy in hydrochloric acid solution [J]. Corros Sci, 2006, 48: 2106-2119.
[6] BARBUCCI A, FARNE G, MATTEAZZI P, RICCIERI R, CERSOLA G. Corrosion behavior of nanocrystalline Cu90Ni10 alloy in neutral solution containing chlorides [J]. Corros Sci, 1999, 41: 463-475.
[7] CAO Zhong-qiu, LIU Wei-hua, ZHENG Zhi-guo, NIU Yan. Corrosion behavior of Cu-40Ni alloy with different grain sizes in acidic media [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(1): 170-175. (in Chinese)
[8] CAO Zhong-qiu, LIU Wei-hua, XUE Rong, ZHENG Zhi-guo. Effect of grain size on corrosion behavior of Cu-40Ni alloys in acidic media containing Cl- [J]. Chinese Journal of Rare Metals, 2006, 30(6): 735-739.
[9] BENJAMIN J S. Dispersion strengthened superalloys by mechanical alloying [J]. Metall Trans, 1970, 1: 2943-2950.
[10] RYU S S, KIM Y D, MOON H I. Dilatometric analysis on the sintering behavior of nanocrystalline W-Cu prepared by mechanical alloying [J]. J Alloys and Compd,2002, 335(1/2): 233-240.
[11] HUANG X S, TSUTOMU, MASHIMO. Nonequilibrium alloy powders and bulk alloys in W-Ag system prepared by mechanical alloying and shock compression [J]. J Alloys Compd, 2003, 361(1/2): 118-124.
[12] WANG C L, LIN S Z, NIU Y, ZHAO Z L, WU W T. Microstructural properties of bulk nanocrystalline Ag-Ni alloy prepared by hot pressing of mechanically pre-alloyed powders [J]. Appl Phys, 2003, A76: 157-163.
[13] FU G Y, NIU Y, GESMUNDO F. Microstructural effects on the high temperature oxidation of two-phase Cu-Cr alloys in 1 atm O2 [J]. Corro Sci,2003, 45(3): 559-574.
[14] MANSFELD F, LIU G, XIAO H, TSAI H, LITTLE B J. The corrosion behavior of copper alloys, stainless steels and titanium in seawater [J]. Corros Sci, 1994, 36: 2063-2095.
[15] ISMAIL K M, FATHI A M, BADAWY W A. Effect of nickel content on the corrosion and passivation of copper-nickel alloys in sodium sulfate solution [J]. Corrosion, 2004, 60(9): 795-803.
[16] CAO Chu-nan. Corrosion electrochemistry [M]. Beijing: Chemical Industry Press, 1990: 94.
(Edited by LI Xiang-qun)
Foundation item: Project(50771068) supported by the National Natural Science Foundation of China; Project(20062049) supported by Natural Science Foundation of Liaoning Province, China
Corresponding author: CAO Zhong-qiu; Tel: +86-24-86593317; E-mail: caozhongqiu6508@sina.com


