Trans. Nonferrous Met. Soc. China 26(2016) 3253-3257
Effect and mechanism of octanol in cassiterite flotation using benzohydroxamic acid as collector
Lei SUN, Yue-hua HU, Wei SUN
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 31 May 2016; accepted 18 October 2016
Abstract:
The effect of octanol in cassiterite flotation using benzohydroxamic acid (BHA) as a collector was investigated. The adsorption mechanism of octanol and BHA on the surface of cassiterite was analyzed by adsorption experiments and infrared spectra analysis. Micro-flotation results indicated that single octanol exhibited almost no collecting power to cassiterite over a wide pH range. However, as an auxiliary collector, octanol could markedly decrease the consumption of collector BHA and keep the recovery of cassiterite in high level. The results of adsorption experiments and infrared spectra demonstrated that single octanol was not adsorbed on the surface of cassiterite. It formed adsorption connected with BHA on the surface of cassiterite, and enhanced the hydrophobicity of cassiterite. Octanol promoted the adsorption amount of BHA on the cassiterite surface, and decreased the consumption of BHA.
Key words:
octanol; benzohydroxamic acid; cassiterite; flotation; mechanism;
1 Introduction
Cassiterite is the primary mineral from which tin metal is extracted. The estimated resource of tin metal is about 4.9×106 t in the world, and 30.5% of it is in China [1]. Because of the characteristics of heavy, hard, and extremely brittle, cassiterite is preferentially concentrated by gravity separators [2-5]. However, the separation efficiency of gravity concentration drops considerably in dealing with the complex polymetallic low-grade cassiterite ore in the form of fine and ultrafine size [6,7].
The development of oxide minerals surfactants makes it possible to recover the fine and ultrafine cassiterite from gravity tailings by flotation [8-14]. SREENIVAS and MANOHAR [15] investigated the flotation and surface chemistry of cassiterite in the presence of octyl hydroxamate. During the pulp pH value from 6.2 to 9.0, octyl hydroxamate showed good flotation ability to cassiterite, and it can be adsorbed on the surface of cassiterite by the combination of physical and specific forces. WU and ZHU [16] investigated selective flotation of cassiterite from synthetic mixtures of cassiterite-quartz and cassiterite-calcite using benzohydroxamic acid (BHA). Laboratory micro- flotation studies showed that BHA floated cassiterite more efficiently than calcite and quartz with the existence of sodium hexametaphosphate. The main interaction between BHA and the surface of cassiterite was chemisorption with the formation of Sn–BHA compounds. SUN et al [17] applied BHA to the flotation of complex polymetallic low-grade cassiterite in Yunnan province of China. The concentrate was upgraded from 0.55% Sn to approximately 40% with the recovery of 65%. Analogously as BHA, salicylhydroxamic acid (SHA) showed good flotation ability to cassiterite, and it can be adsorbed on the surface of cassiterite by a formation of Sn-SHA compounds [18,19].
Although the surfactants mentioned above achieved good results on cassiterite flotation in the laboratory, BHA is most widely used as a collector in the cassiterite flotation industry at present. However, the dosage of BHA is usually more than 1 kg/t ore, which is a quite high cost for production. In this work, the influence of octanol in cassiterite flotation was investigated to reduce the dosage of BHA. The adsorption mechanism of octanol and BHA on the surface of cassiterite was analyzed by ultraviolet spectroscopy and infrared spectroscopy measurements.
2 Experimental
2.1 Materials
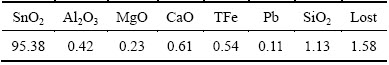
The high-grade cassiterite samples used in the experiments were collected from Yunnan province, China. After being further purified by gravity concentration, the samples were ground into powders, and then analyzed by X-ray diffraction (Fig. 1) and chemical composition (Table 1). It indicated that most of the samples were pure cassiterite with the purity of SnO2 over 95%. The samples with the particle size below 74 μm were used in micro-flotation tests and ultraviolet spectroscopy measurements, and the samples with the particle size below 5 μm were used in infrared spectroscopy measurements. The purity of the BHA used in the experiments was over 98%, and octanol was spectral pure. Analytical grade sodium hydroxide and hydrochloric acid were used for mineral pulp pH control. All the reagents were dissolved in deionized water before being used in the experiments each time.
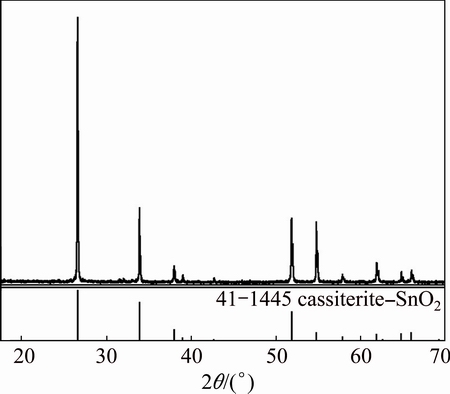
Fig. 1 XRD pattern of cassiterite samples
Table 1 Chemical composition of cassiterite samples (mass fraction, %)
2.2 Micro-flotation tests
Micro-flotation tests were carried out in a 40 mL flotation cell. The impeller speed was fixed at 1650 r/min. In each test, 3.0 g of cassiterite was dispersed in 30 mL deionized water. After adjusting the pulp pH to the desired value with a INESA PHS-3C pH meter, the flotation collector was added into the cell in the desired amount, then the pulp was mixed for 3 min in order to disperse the collector and cassiterite well. The flotation was carried out for 4 min, and the float froth and the pulp fraction were collected and dried separately. Then, each dried solid fraction was weighed and used to calculate the recovery of cassiterite.
2.3 Adsorption
The BHA solution samples at concentrations of 0.1, 0.2, 0.3 and 0.4 mmol/L were prepared for calculating the working curve. In each test, 2.0 g of cassiterite was dispersed in 30 mL BHA and BHA + octanol solution of known concentration at different pH values separately, and mixed for 20 min at 1000 r/min. Then, solid-liquid separation was carried out by centrifuging the suspension in a high speed centrifuge at 5000 r/min for 15 min. The separated liquid was adjusted to pH 7.0, and determined the concentration of BHA by a SHIMADZU UV-2600 ultraviolet-visible spectrophotometer. All experiments were performed in a temperature-controlled water bath at 298 K. The adsorption density of BHA on the surface of cassiterite was calculated by
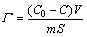 (1)
(1)
where Γ is the adsorption density of BHA on the surface of cassiterite (mol/m2), C0 is the initial concentration of BHA (mol/L), C is the residual concentration of BHA (mol/L), V is the volume of solution (L), m is the mass of cassiterite (g), and S is the specific surface area of cassiterite (m2/g).
2.4 Infrared spectroscopy
The infrared spectra of samples including BHA, octanol, cassiterite, and their interaction products (cassiterite+BHA, cassiterite+octanol, and cassiterite+ BHA+octanol) were tested on a SHIMADZU IRAffinity-1S Fourier transform infrared spectrometer in this experiment to characterize the nature of the interactions between the collectors and cassiterite. The interaction products were firstly prepared by contacting cassiterite samples (<5 μm) with BHA and octanol solution at pH 7.0, then washed with distilled water three times and dried at the temperature of 298 K. Infrared spectra were carried out with a KBr disk that contained 0.5% of the required sample to scan in the wave number range of 4000 to 500 cm-1 at a resolution of 4 cm-1.
3 Results and discussion
3.1 Micro-flotation tests of cassiterite
BHA and octanol were used as collectors separately in the micro-flotation tests of cassiterite. The concentrations of both collectors were 0.6 mmol/L. The recovery of cassiterite as a function of pulp pH value is presented in Fig. 2. The results showed that using BHA as the collector, the recovery of cassiterite increased as a function of pulp pH value from 2 to 7, and decreased sharply with further increase of pulp pH value from 7 to 12. The maximum recovery was about 85% at pH 7.0. Using octanol as the collector, the recovery of cassiterite was less than 5% in the pH range of 2 to 12.
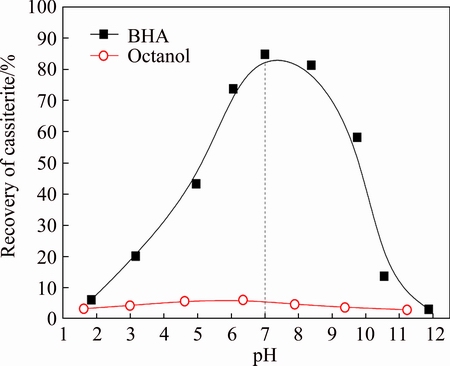
Fig. 2 Flotation recovery of cassiterite as function of pulp pH value using BHA and octanol as collectors
The flotation recovery of cassiterite as a function of concentration of BHA and octanol at pH 7.0 is shown in Fig. 3. Using BHA as the collector, the recovery of cassiterite increased as a function of concentration of BHA from 0 to 0.6 mmol/L, and reached 85% at concentration of 0.6 mmol/L. When the concentration of BHA was more than 0.6 mmol/L, the flotation recovery of cassiterite increased insignificantly. The concentration of 0.6 mmol/L was defined as an inflection point concentration. Using octanol as the collector, the recovery of cassiterite was less than 10% as a function of concentration of octanol from 0 to 0.9 mmol/L.
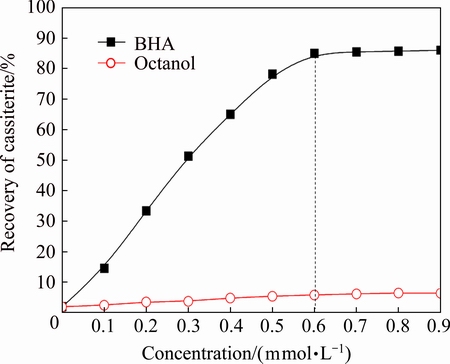
Fig. 3 Flotation recovery of cassiterite as function of concentration of BHA and octanol at pH 7.0
Figures 2 and 3 demonstrate that BHA performed an excellent collecting power for cassiterite in the pulp pH value ranging from 7 to 9. Single octanol exhibited almost no collecting power for cassiterite over a wide pH range.
The flotation recovery of cassiterite as a function of concentration of BHA with different concentrations of octanol at pulp pH 7.0 is summarized in Fig. 4. As the concentration of octanol increasing from 0 to 0.06 mmol/L, the inflection point concentration of BHA decreased from 0.6 to 0.3 mmol/L. Specifically, addition of octanol as an auxiliary collector with the concentration of 0.06 mmol/L, the recovery of cassiterite reached 82% with 0.3 mmol/L BHA at pH 7.0. This indicates that although single octanol exhibited almost no collecting power for cassiterite, it could markedly decrease the consumption of BHA and keep the recovery of cassiterite in high level.
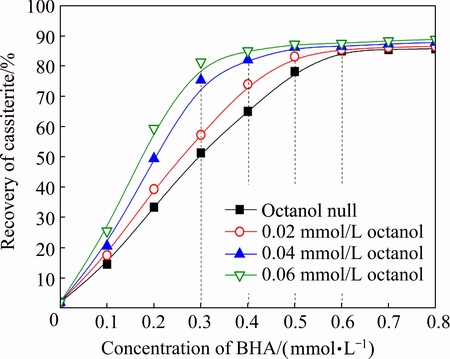
Fig. 4 Flotation recovery of cassiterite as function of concentration of BHA with different concentrations of octanol at pH 7.0
3.2 Adsorption of BHA on cassiterite
In BHA solution, the ultra violet absorbance around 227 nm was confirmed according to the Lambert-Beer law in the range from 0.1 mmol/L to 0.4 mmol/L at pH 7.0, and the square correlation coefficient of working curve (R2) was 0.9996. The adsorption amount of BHA on the cassiterite surface as a function of pH value with different concentrations of collectors is presented in Fig. 5.
The results demonstrated that all the maximum adsorption of BHA occurred around pH 7.0. Furthermore, when the initial concentration of BHA was 0.2 mmol/L, the maximum adsorption amount of BHA was 0.0026 mmol/g at pH 7.0. Keeping the initial concentration of BHA unchanged, the maximum adsorption amount of BHA increased to 0.0052 mmol/g with 0.04 mmol/L octanol at pH 7.0, which was close to the maximum adsorption amount of BHA in the concentration of 0.4 mmol/L at pH 7.0. This indicated that octanol could promote the adsorption of BHA on the cassiterite surface. The absorption results were consistent with the micro-flotation experiments.
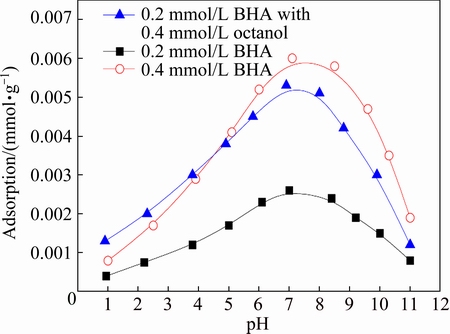
Fig. 5 Adsorption amount of BHA on cassiterite as function of pH value
3.3 Infrared spectroscopy analysis
The infrared spectra of octanol, cassiterite, and cassiterite treated with octanol (cassiterite+octanol) are presented and compared in Fig. 6. The infrared spectra of octanol showed that the adsorption peak around 3327 cm-1 was due to O—H stretching vibration adsorption peak, the adsorption peak around 2927 cm-1 was due to C—H stretching vibration adsorption peak, and the adsorption peak around 1059 cm-1 was due to C—O stretching vibration adsorption peak. The characteristic peaks of cassiterite were around 654 and 568 cm-1. Compared with the infrared spectrum of octanol and cassiterite, cassiterite+octanol exhibited the same infrared spectrum as cassiterite. This indicated that octanol was not adsorbed on the surface of cassiterite, and this confirmed the micro-flotation results of cassiterite using octanol as the collector.
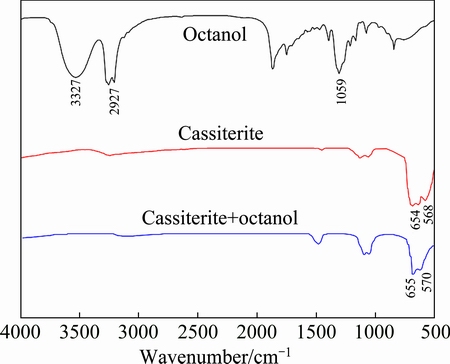
Fig. 6 Infrared spectra of octanol, cassiterite and cassiterite treated with octanol
The infrared spectra of BHA, octanol, and cassiterite treated with BHA and octanol (cassiterite+ BHA+octanol) are presented and compared in Fig. 7. The infrared spectra of cassiterite+BHA+octanol showed that the O—H stretching vibration peak of octanol, shifted from 3327 to 3377 cm-1, was due to the possible formation of a hydrogen bond (O—H…N) with BHA. The stretching vibration peaks around 2895 and 1176 cm-1 were shifted from 2927 and 1059 cm-1, which were due to stretching vibration adsorption peaks of octanol. Appearance of the octanol characteristic adsorption peaks indicated that octanol was adsorbed on the surface of cassiterite with BHA [12,16,20,21]. Adsorption experiments and infrared spectra analysis demonstrated that octanol formed electrostatic adsorption with the BHA on the surface of cassiterite, not just promoting the adsorption of BHA. This is consistent with the cassiterite micro-flotation result using BHA as the collector and octanol as the auxiliary collector. Based on these analyses, a potential formation of BHA and octanol adsorbed on the surface of cassiterite is suggested in Fig. 8.
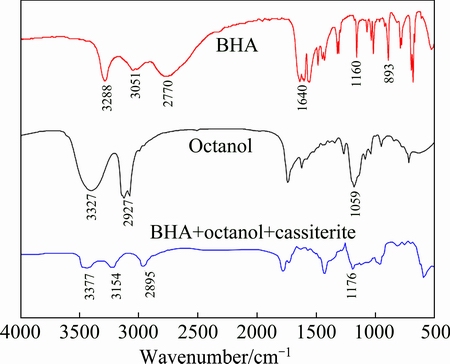
Fig. 7 Infrared spectra of BHA, octanol and dry cassiterite treated with BHA and octanol
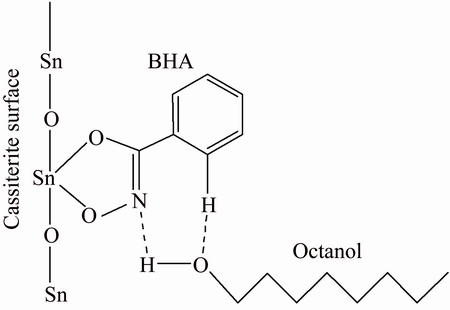
Fig. 8 Potential formation diagram of BHA and octanol adsorbed on cassiterite surface
4 Conclusions
1) Micro-flotation results indicated that octanol exhibited almost no collecting power for cassiterite over a wide pH range, but as an auxiliary collector, it could markedly decrease the consumption of collector BHA and keep the recovery of cassiterite in high level at pH 7.0.
2) The results of adsorption experiments and infrared spectra demonstrated that single octanol was not adsorbed on the surface of cassiterite. Octanol formed adsorption connected with the BHA, and promoted the adsorption amount of BHA on the surface of cassiterite. These were two aspects of mechanisms for decreasing the consumption of BHA and keeping the collecting power of BHA in high level. This was confirmed by the cassiterite micro-flotation results. Based on these analyses, a potential formation of BHA and octanol adsorbed on the surface of cassiterite was suggested.
References
[1] ANGADI S I, SREENIVAS T, JEON H S, BAEK S H, MISHRA B K. A review of cassiterite beneficiation fundamentals and plant practices [J]. Minerals Engineering, 2015, 70: 178-200.
[2] FALCONER A. Gravity separation: Old technique/new methods [J]. Physical Separation in Science and Engineering, 2003, 12(1): 31-48.
[3] KEMENADE E, MONDT E, HENDRIKS T, VERBEEK P. Liquid-phase separation with the rotational particle separator [J]. Chemical Engineering & Technology, 2003, 26(11): 1176-1183.
[4] KROLL-RABOTIN J S, BOURGEOIS F, CLIMENT  . Physical analysis and modeling of the Falcon concentrator for beneficiation of ultrafine particles [J]. International Journal of Mineral Processing, 2013, 121: 39-50.
. Physical analysis and modeling of the Falcon concentrator for beneficiation of ultrafine particles [J]. International Journal of Mineral Processing, 2013, 121: 39-50.
[5] YANG Wei-lin, DAI Hui-xin, WANG Hong-jun. Progress of cassiterite sulfide ore beneficiation [J]. Applied Mechanics and Materials, 2014, 644-650: 5439-5442.
[6] ZHAO Yue-min, WEN Xue-feng, SHI Hong-xia, JIAO Hong-guang, TAO You-jun. Study on metals recovery from -0.074 mm printed circuit boards by enhanced gravity separation [J]. The Chinese Journal of Process Engineering, 2006(2): 201-204.
[7] WANG X S, MILES N J, KINGMAN S. Numerical study of centrifugal fluidized bed separation [J]. Minerals Engineering, 2006, 19(10): 1109-1114.
[8] GRUNER H, BILSING U. Cassiterite flotation using styrene phosphonic acid to produce high-grade concentrates at high recoveries from finely disseminated ores [J]. Minerals Engineering, 1992, 5: 429-434.
[9] BULATOVIC S, de SILVIO E. Process development for impurity removal from a tin gravity concentrate [J]. Minerals Engineering, 2000, 13(8-9): 871-879.
[10] HAY M P, RULE C M. SUPASIM: A flotation plant design and analysis methodology [J]. Minerals Engineering, 2003, 16(11): 1103-1109.
[11] LAN Zhuo-yue, ZHOU Yong-cheng, TONG Xiong. Recovery of fine cassiterite from tin tailings slime by froth flotation [J]. Advanced Materials Research, 2013, 634-638: 3478-3483.
[12] WANG Pei-pei, QIN Wen-qing, REN Liu-yi, WEI Qian, LIU Rui-zeng, YANG Cong-ren, ZHONG Shui-ping. Solution chemistry and utilization of alkyl hydroxamic acid in flotation of fine cassiterite [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(6): 1789-1796.
[13] ZHOU Yong-cheng, TONG Xiong, SONG Shao-xian, WANG Xiao, DENG Zheng-bin, XIE Xian. Beneficiation of cassiterite fines from a tin tailing slime by froth flotation [J]. Separation Science and Technology, 2014, 49: 458-463.
[14] LIANG Yi-qiang, JIAN Sheng, ZHU Cong-jie, QIAO Ji-bo. Study on the mineral processing of cassiterite from flotation tailings by a combined method [J]. Advanced Materials Research, 2014, 968: 185-189.
[15] SREENIVAS T, MANOHAR C. Adsorption of octyl hydroxamic acid/salt on cassiterite [J]. Mineral Processing and Extractive Metallurgy Review, 2008, 20: 503-519.
[16] WU Xi-qing, ZHU Jian-guang. Selective flotation of cassiterite with benzohydroxamic acid [J]. Minerals Engineering, 2006, 19(14): 1410-1417.
[17] SUN Wei, KE Li-fang, SUN Lei. Study of the application and mechanism of benzohydroxamic acid in the flotation of cassiterite [J]. Journal of China University of Mining &Technolog, 2013, 42(1): 62-68. (in Chinese)
[18] QIN Wen-qing, XU Yang-bao, LIU Hui, REN Liu-yi, YANG Cong-ren. Flotation and surface behavior of cassiterite with salicylhydroxamic acid [J]. Industrial & Engineering Chemistry Research, 2011, 50(18): 10778-10783.
[19] QIN Wen-qing, REN Liu-yi, XU Yang-bao, WANG Pei-pei, MA Xi-hong. Adsorption mechanism of mixed salicylhydroxamic acid and tributyl phosphate collectors in fine cassiterite electro-flotation system [J]. Journal of Central South University, 2012, 19(6): 1711-1717.
[20] ZHU Hai-ling, DENG Hai-bo, CHEN Chen. Flotation separation of andalusite from quartz using sodium petroleum sulfonate as collector [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1279-1285.
[21] LIU Rui-zeng, QIN Wen-qing, JIAO Fen, WANG Xing-jie, PEI Bin, YANG Yong-jun, LAI Chun-hua. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(1): 265-271.
辛醇对苯甲羟肟酸浮选锡石的影响和机理
孙 磊,胡岳华,孙 伟
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:研究了辛醇对苯甲羟肟酸(BHA)浮选锡石纯矿物的影响, 分析了辛醇和BHA在锡石表面的吸附机理。实验室浮选实验结果表明,在广泛pH区间内,单一辛醇对锡石没有浮选性,但是作为辅助捕收剂,辛醇可以显著降低捕收剂BHA的用量,并且使锡石仍保持较高回收率。红外光谱和吸附量研究表明,单一辛醇在锡石表面没有发生吸附,但是辛醇与BHA形成吸附组合体而吸附在锡石表面,增强了锡石表面的疏水性;同时辛醇使BHA在锡石表面的吸附量增加,从而降低了BHA的用量。
关键词:辛醇;苯甲羟肟酸;锡石;浮选;机理
(Edited by Xiang-qun LI)
Foundation item: Project (B14034) supported by the National “111” Project of Ministry of Education of China; Project (2015CX005) supported by the Innovation Driven Plan of Central South University, China; Project supported by the 2014 Sublimation Scholar Program of Central South University, China
Corresponding author: Yue-hua HU; Tel: +86-731-88836873; E-mail: hyh@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64458-8
Abstract: The effect of octanol in cassiterite flotation using benzohydroxamic acid (BHA) as a collector was investigated. The adsorption mechanism of octanol and BHA on the surface of cassiterite was analyzed by adsorption experiments and infrared spectra analysis. Micro-flotation results indicated that single octanol exhibited almost no collecting power to cassiterite over a wide pH range. However, as an auxiliary collector, octanol could markedly decrease the consumption of collector BHA and keep the recovery of cassiterite in high level. The results of adsorption experiments and infrared spectra demonstrated that single octanol was not adsorbed on the surface of cassiterite. It formed adsorption connected with BHA on the surface of cassiterite, and enhanced the hydrophobicity of cassiterite. Octanol promoted the adsorption amount of BHA on the cassiterite surface, and decreased the consumption of BHA.


