
Synthesis and characterization of tantalum nitride nanopowder prepared through homogeneous reaction
MA Chun-hong(马春红)1, 2, ZHANG Wei-feng(张威峰)1, HE Ji-lin(何季麟)2, ZHU Hong-min(朱鸿民)1
1. School of Metallurgical and Ecological Engineering,
University of Science and Technology Beijing, Beijing 100083, China;
2. Ningxia Orient Tantalum Industry Ltd., Shizuishan 753000, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Tantalum nitride powders with particle size of 20-50 nm were prepared by the homogeneous reduction in liquid ammonia, and were treated at the temperature range from 500 ℃ to 1 170 ℃. The results of XRD analysis indicate that the powders heated up to 500 ℃ are in the form of amorphous. The powder heated at 600 ℃ tends to transform into crystal, the powder heated to temperatures higher than 700 ℃ is clearly of crystal. The results of surface area analysis by BET show that the surface area of the powder increases as the heating temperature increases, and reaches a maximum value of 21.8 m2/g at the heating temperature around 700 ℃.
Key words:
homogeneous reduction; tantalum nano powder; BET surface area analysis;
1 Introduction
The method for production of tantalum metal powder for tantalum capacitors in large scale was developed by HELLIER and MARTIN in 1950s, and was improved by the following research. Potassium heptafluorotantalate (K2TaF7) mixed with diluent salt typically NaCl was reduced by sodium in the process. The reaction temperature is 800-900 ℃[1-2].
K2TaF7+5Na→Ta+5NaF+2KF+heat (1)
This strongly exothermic reaction is controlled by adding inert salts such as KCl, NaCl, KF, NaF to the reduction mixture[3-4]. In order to achieve isothermal conditions one of the reactants (i.e. Na) is carefully added with controlled heat removal.
The highest capacitance value of Ta powder commercially manufactured through reduction of K2TaF7 by sodium is 100 000-150 000 μF?V/g[5-6].
In recent years SHEKHTER et al obtained tantalum powder with fine particle size through reducing Ta2O5 with gaseous Mg. The reaction temperature is 1 000 ℃. The original particle size is 100-200 nm. The BET area of the Ta powder is 2-2.5 m2/g. And its capacitance is 100 000-150 000 μF?V/g[7-9].
Ta2O5+5Mg→2Ta+5MgO+heat (2)
“Broccoli”-like morphology of Ta powder was found when Ca reduces Ta2O5 in the molten CaCl2. The reaction temperature is 950 ℃. It consists of fine particles and branches, and it is different from the conventional spherical particles. The BET area of the Ta powder is 0.9-1.6 m2/g, and its capacitance is 60 000 μF?V/g[10-11].
Ta2O5+Ca(in CaCl2)→Ta+CaO( in CaCl2) (3)
Reactions (1)-(3) perform at high temperature. And it is difficult to obtain finer powder due to the high reaction temperature under which the particles grow easily.
Homogeneous reaction in liquid ammonia provides a new route to synthesize the nanosize-powder with high surface area. The products by the reaction of solutions of tantalum pentachloride with sodium are sodium chloride and nanosize-powder in amorphous structure[12-13]. The diameter of the particles is 20-60 nm.
TaCl5+5Na→Ta(s)+5NaCl (4)
However, the product powder through this route consists of large content of nitrogen. In this paper, we studied the possibility of synthesizing the tantalum nitride with particle size in the order of several tens of nano-meters, and with large surface area. The powder produced with reduction is heated at various temperatures from 500 ℃ to 1 170 ℃. The morphology and structure of the powder are studied by XRD, TEM, and BET surface area measurement.
2 Experimental
2.1 Preparation of powder
Tantalum halide ( TaCl5, 15 g ) and sodium (5.5 g , in stoichiometric excess by 2% to 20% on a molar basis) were respectively charged into Reactor A and B made from borosilicate glass in an argon-filled glove box (MECAPLEX, Switzerland, O≤2×10-6). The reactors were removed from the glove box, and connected to a vacuum system. The reactor with TaCl5 was infused with ammonia gas for several hours at room temperature. And then the reactors were immersed in a cryostat (model FP50-MV, Julabo, Germany) maintained at -45 ℃, which is lower than the boiling point of ammonia (-33.8 ℃). Ammonia gas was condensed into liquid. After sodium and TaCl5 were dissolved in liquid ammonia, the salt-ammonia solution was pressed by aerating argon into the blue sodium-ammonia solution through a tube between reactors in several steps. A fine black powder precipitated at the bottom of Reactor B. Then the temperature of the cryostat was raised slowly. This promoted the evaporation of ammonia from Reactor B. After all the ammonia had evaporated, the product was taken out in the glove box, and was put into a filter fixed in a Soxhlet extraction tube. Liquid ammonia was used as the solvent and the extraction continued for over 40 h. The black powder was retained in the filter, and the white powder was extracted in a flask.
2.2 Heating treatment at different temperatures
The powder obtained by the reduction was then heated under vacuum at various temperatures. The powder was put into a tantalum crucible, and then was placed into the quartz tube. The operation was done in the glove-box under the atmosphere of argon. The quartz tube was sealed, and moved out from the glove box, then was placed into the furnace and connected with the vacuum system. The powder was kept under vacuum, and the temperature of the furnace was raised to set value, and kept for 2 h.
2.3 Analysis
1) The powders with and without heat-treatment were characterized by transmission electron microscopy (JEOL TEM-200CX, Japan JEOL Co.).
2) Nitrogen content in the black powder treated at 900 ℃ was measured by pulse-heating inert-gas fusion thermoconducting method (Model TC-436 nitrogen/ oxygen determinator, LECO Corp.). Tantalum content was measured by gravimetry method.
3) The sample was analyzed by XRD (MXP21VAHF, MAC Science Co. Ltd Japan) in order to analyze the structure of the powders heat-treated at various temperatures.
4) The surface area of the powders was tested by high speed auto-analyzer of the surface area and porosity (NOVA4000, America).
3 Results and discussion
Fig.1 shows the TEM images of the powders obtained after ammonia extraction, without heat treatment (a), and heat-treated at 900 ℃(b). The particle size of the powder in the range of 20-50 nm. The powder without heat treatment is somewhat porous. No distinct difference exist in the particle size of powder, with different heat treatment temperatures up to 900 ℃.
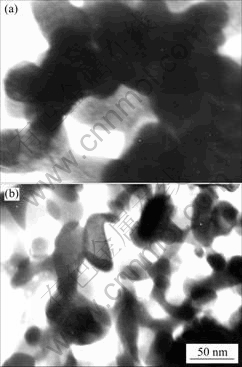
Fig.1 TEM images of powder without heat treatment (a) and heat-treated at 900 ℃ (b)
Fig.2 shows the X-ray diffraction patterns of the powders after heat-treated at 500, 600, 700, 800, 1 000 and 1 170 ℃ respectively. The XRD patterns of powder heated at temperatures lower than 500 ℃ show typical pattern of an amorphous structure. In the XRD pattern of the powder heated at 600 ℃, weak crystal peaks are observed. It is indicated that the crystallization of micro-structure starts around this temperature. The powder heated at temperatures higher than 700 ℃ shows clear crystal XRD patterns. In the same figure, the pattern is compared with the standard XRD pattern of cubic TaN (card JCPS-1993, 49-1283). From Fig.2, it is clear that the main XRD peaks of the product powder are consistent with those of TaN standard. As a result of the reaction performed in liquid ammonia, the product absorbed large quantity of ammonia becomes into tantalum nitride after being heated.
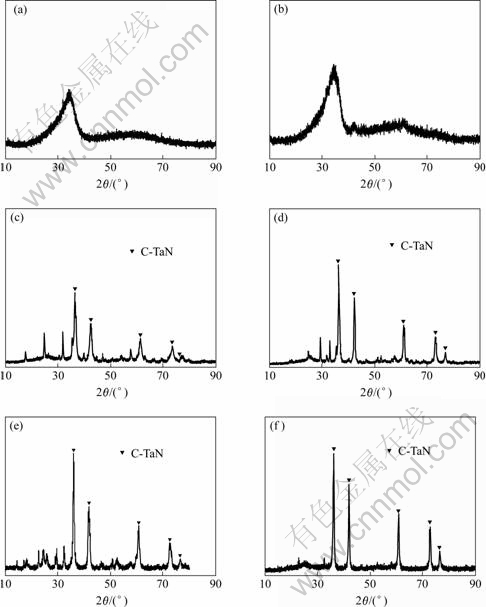
Fig.2 XRD patterns of powders after heat treatments at 500 (a), 600 (b), 700 (c), 800 (d), 1 000 (e), 1 170 ℃ (f), respectively
The typical chemical contents of powder after being heated at 900 ℃ are tantalum 89.0% and nitrogen from 6.5% to 8.0%. This is equivalent to the molar ratio of Ta/N, which is from 1?0.95 to 1?1.16. Clearly, the results of chemical analysis are consistent with that of XRD analysis.
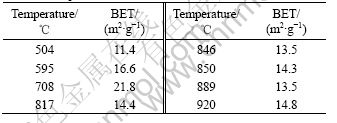
Table 1 shows the surface area of the powders heated at various temperatures. The surface area of the powder is all bigger than 11 m2/g. Obviously, the tantalum nitride powder obtained through this route is very fine. It is interesting that surface area measured by BET increases as the heating temperatures increase, and reaches the maximum at the temperature of around 700 ℃. The surface area of the powder heated at 700 ℃ reaches 21 m2/g. We do not have a clear explanation on this. It may have some relation with the crystallization of microstructure, since the powder becomes into a crystal at exactly the same temperature (700 ℃).
Table 1 Surface area of powders after heat-treatment at different temperatures
4 Conclusions
1) The particle size of tantalum nitride powders without and with heat-treatment under 900 ℃ is 20-50 nm. The particle size is almost the same up to this temperature.
2) The morphology of the powder prepared by homogeneous reaction begins to transform into crystal after heat-treated at 600 ℃. The product heated at temperatures higher than 700 ℃ turns into cubic TaN.
3) The BET surface area of Ta powder prepared by homogeneous reaction after heat-treatment is higher than 13 m2/g.
References
[1] Hellier E G, Martin, G L. Production of tantalum powder. US 2950185[P]. 1960.
[2] Tripp T B. The production of tantalum by the sodium reduction process[C]//Symposium on Ta and Nb Proceedings. 31st TIC, 1990: 256-259.
[3] Habashi F. Handbook of extractive metallurgy[M]. Wiley-VCH, 1997: 1417.
[4] Tripp T B, Eckert J. Kirk-othmer encyclopedia of chemical technology[M]. 4th ed. 1997: 658.
[5] LIU Hong-dong, PAN Lun-tao, LU Zhen-da. Progress in high capacity Ta powder[J]. Rare Metals, 2003, 27(1): 35-38.
[6] HE Ji-lin. The development of world tantalum powder technology[J]. Engineering Science China, 2001, 3(12): 85-89. (in Chinese)
[7] Shekhter L, Lanin L, Tripp T, Goldberg H. A new process for the reduction of tantalum and niobium powder from oxide[C]//Int Symposium on Ta and Nb Proceedings, 41st TIC. 2000: 87-102.
[8] Shekhter L N, Tripp T B, Lanin L L. Metal powders produced by the reduction of the oxides with gaseous magnesium. PCT Patent WO 00/67936[P]. 2000.
[9] Shekhter L N, Tripp T B, Lanin L L. Method for producing tantalum/niobium metal powders by the reduction of their oxides with gaseous magnesium. US 6171363[P]. 2001.
[10] Ryosuke O S, Masahiko B, Youhei O, Kosuke Y. Formation of broccoli-like morphology of tantalum powder[J]. J Alloys Compd, 2005, 389: 310-316.
[11] Masahiko B, Ryosuke O S. Dielectric properties of tantalum powder with broccoli-like morphology[J]. J Alloys Compd, 2005, 392: 225-230.
[12] Zhu Hong-min, Sadoway D R. Synthesis of nanoscale particles of Ta and Nb3Al by homogeneous reduction in liquid ammonia[J]. J Mater Res, 2001, 16(9): 2544-2549.
[13] Zhu Hong-min, He Ji-lin, Cao Zhan-min. Nanopowder of tantalum prepared through homogeneous reaction[J]. Metallurgy Rearch, 2003: 356-359.
Foundation item: Project(2003AA302430) supported by the National Hi-tech Research and Development Program of China; project(50374007) supported by the National Natural Science Foundation of China
Corresponding author: ZHU Hong-min; Tel: +86-10-62332267; E-mail: hzhu@metall.ustb.edu.cn
(Edited by YUAN Sai-qian)


