
Influence of additive element on surface oxide film of A356 alloy
OUYANG Zhi-ying(欧阳志英), LIANG Hong-yu(梁红玉), MAO Xie-min(毛协民), HONG Mei(红 梅)
School of Material Science and Engineering, Shanghai University, Shanghai 200072, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
The influences of RE-modification and Sr-modification on the hydrogen content and surface oxide film of A356 aluminum alloy melt were investigated. The hydrogen content of the melt was measured by reduce pressure test. The phases in the surface oxide film were analyzed by X-ray diffractometry (XRD), and the morphology of the surface oxide film was observed by scanning electronic microscopy (SEM). The results show that RE-modification reduces the hydrogen content of A356 aluminum alloy greatly. Contrarily, Sr-modification increases the hydrogen content remarkably. After being treated with RE, a large number of LaAl11O18 consisting of Al2O3 and La2O3, are generated in the surface oxide film of A356 alloy. The surface oxide film of Sr-modification is almost composed of Al2SrO4. According to the results of SEM, the surface oxide film of Sr-modification is very easy to crack, destroy the continuity and compactness of surface oxide film, accelerate the vapor diffusing into the melt, consequently, increase the hydrogen content of A356 alloy melt significantly. But RE-modification makes the surface oxide film compact, and restrains the aluminum exposed to water, so reduces the hydrogen content of A356 alloy melt.
Key words:
A356 aluminum alloy; rare earth elements; surface oxide film; gas porosity; strontium;
1 Introduction
It is commonly known that the strength and quality of an Al-Si alloy casting are determined by its microstructure and the amount of porosity present in the casting. Modification is one of the processes used to improve the microstructure quality. For Al-Si alloy, Sr and rare earth (RE) are used extensively because of their excellent modification effect. Many experiments have been carried out to study and explain the effect of modifier on hydrogen content and porosity. Generally, it was considered that Sr-modification was associated with an increase tendency to porosity formation [1-3], and RE-modification would reduce the porosity in the casting [4-8]. However, there is no consensus on the mechanism. Some possible reasons have been proposed and studied. In general, modifiers can increase or decrease the inclusion content in the melt, decrease or increase hydrogen solubility in solid metal, increase or decrease the volumetric shrinkage, and change the solid/liquid interface morphology[9-12].
It is well known that there exists a surface oxide film above the aluminum melt, and if the oxide film is compact, this film can prevent the vapor and oxygen from diffusing into the melt, resulting in a cleaner melt and low hydrogen level. But if it isn’t, then this film can’t protect the melt from acting with those gases, and would increase the hydrogen content in the melt. So, the integrity, compactness, microstructure and thickness of this film may have important impact on the hydrogen content of the melt[13]. Although some people have hypothesized the effect of modifier on the hydrogen maybe is due to they changed the structure of surface oxide film, but lack of direct record to prove it [14]. The purpose of this work is to investigate the effect of modifier on the surface oxide film, and explain the mechanics of the modifier on the melt hydrogen content.
2 Experimental
A356 aluminum alloy was used as a base alloy, Sr (added as Al-10%Sr master alloy) and RE (added as Al-10%RE master alloy) were added to the base alloy to study their effect on the hydrogen level and surface oxide film. The mother alloy was melted at 740 ℃ in a graphite crucible. When the alloy was completely melted, and the temperature was suitable, added modifier, gently stirred, held for about 10 min, then degassed it with N2, cleared off dross and adjusted temperature. Then the melt was poured into nickel crucible (d40 mm×25 mm) at 710 ℃.
Hydrogen content of melt was measured by reduce pressure test, which was used to determine the hydrogen content. The working pressure adapted is 8 kPa, the measure can be finished about 4 min.
The surface oxide film was achieved using a self-designed device. It is composed of mild steel. When the melt was suitable, pressed the pre-heated device into the melt gently, the melt entered into it through the small cavity on the bottom of the device. Hold for 20 min to provide enough time for the oxide film to grow. Then pulled it up, when the oxide film solidified, taken it out. Then analyzed the phases of them by X-ray diffractometry (XRD), and observed the morphology by scanning electronic microscopy (SEM).
3 Results and discussion
Fig.1 shows the hydrogen content of the melt versus holding time for unmodified, Sr-modified and RE-modified melt. It can be seen that, compared with unmodified melt, Sr-modification increases the hydrogen content remarkably, and the hydrogen content was increased linearly with time. After held for 0.5 h, the hydrogen content of melt is increased about 250%. Contrarily, RE-modification reduces the hydrogen content greatly, and slows the ascend rate of melt hydrogen content with hold time. After held for 2 h, the melt hydrogen content reaches to the lowest value.
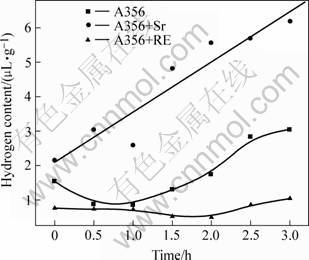
Fig.1 Variation of hydrogen contents vs holding time
Fig.2 shows the photos of the cross-sectional porosity of the reduce press test samples. It can seen that, compared with the unmodified sample, the gas porosities in the Sr-modified sample are larger; some of them even linked together, and the pinhole amount increased greatly. However, there only exist some smaller pinholes in the RE-modified sample even the melt was hold for 2 h.
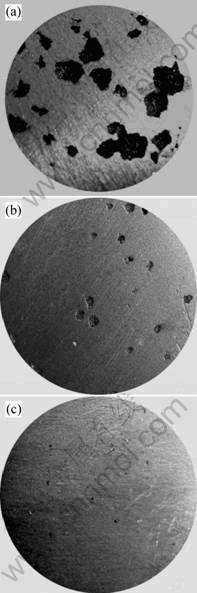
Fig.2 Photos of cross-sectional gas porosity in reduced press test samples: (a) Sr-modified, held for 0.5 h; (b) Unmodified, held for 0.5 h; (c) RE-modified, held for 2 h
It was proved that the hydrogen in the melt mainly comes from the following reaction[14]: Al + H2O→Al2O3+6[H]. Therefore, the melt hydrogen content mainly depends on the amount of H2O, which can diffuse into the melt through the surface oxide film. Apparently, the structure of the surface oxide film is the key factor affecting the hydrogen content of the melt. The better the protective effect is, the lower level the hydrogen content is.
Fig.3 shows the SEM images of the RE-modified and Sr-modified surface oxide film. It can be seen that, the addition of Sr destroys the surface’s integrity and compactness. We can clearly see the crack trace in the Sr-modified surface oxide film (arrow in Fig.3(a)), and the width of the crack reaches to above 100μm. So aluminum is easily exposed to air through these cracks, which promotes the aluminum to react with gases like oxygen and vapor, resulting in a high level melt hydrogen content. Comparatively, the surface oxide film of RE-modified melt is compacter. There exists almost no crack in the film (Fig.3(b)). This structure reduces the probability of aluminum direct contacting with air, and enhances the resistance of gases diffusing into the aluminum melt, then reduces the hydrogen content greatly.
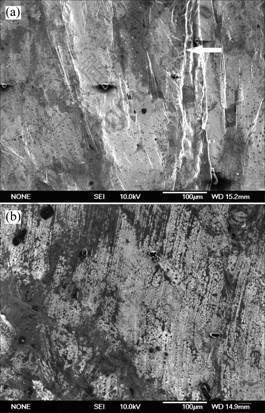
Fig.3 SEM image of surface oxide film layer of Sr-modified melt and RE-modified A356 melt at 710 ℃: (a) A356+ 0.15%Al-10Sr; (b) A356+2.5%Al-10RE
In addition, the phase of the surface oxide film was analyzed by XRD. Fig.4 shows the XRD patterns of the RE-modified and Sr-modified surface oxide film. It is found that, after being treated with RE, a large number of LaAl11O18, which consists of Al2O3 and La2O3, is generated on the surface oxide film. And the Sr-modified surface oxide film is almost composed of Al2SrO4, which consists of SrO and La2O3. So, it is concluded that both Sr and RE change the component of the surface oxide film.
It is well known that La is a surface-active element and is likely to rich at the melt surface. Because its chemical activity is higher than that of aluminum, La would be oxidized by the oxygen preferentially, leading to the barren of La, then the aluminum is oxidized and Al2O3 is generated below the La2O3. Consequently, the compound oxide layer of Al2O3 and La2O3 is formed. This compound oxide film is compacter than Al2O3 [15], increases the resistance of vapor diffusing into aluminum through oxide film. Similarly, Sr is also a surface-active element, and its chemical activity is also higher than that of aluminum, so it will lead to the formation of compound oxide layer of Al2O3 and SrO. But this compound oxide film is looser than Al2O3, because the compacting factor(α) of SrO is only 0.67[16]. In another word, Sr-modification weakens the protective effect of surface oxide film and accelerates the diffusing rate of vapor into aluminum, therefore, increases the hydrogen content remarkably and makes the hydrogen content enhance linearly with the hold time.

Fig.4 XRD patterns of surface oxide film layer of Sr-modified melt and RE-modified A356 melt at 710℃: (a) A356+ 0.15%Al-10Sr; (b) A356+2.5%Al-10RE
4 Conclusions
1) Sr-modification enhances the hydrogen content of A356 aluminum melt notably, resulting in the generation of large amount of round pinholes in the reduce press test sample. Contrarily, RE-modification decreases the hydrogen content of A356 aluminum melt.
2) The addition of Sr makes the surface oxide film more likely to crack, aluminum can be easily exposed to vapor in the air through the crack. However, the addition of RE makes the surface oxide film compacter, and enhances the protective effect of surface oxide film.
3) Modification changes the phases of surface oxide film, the Sr-modification surface oxide film is almost composed of SrAl2O4 and the RE-modification one consists of LaAl11O8.
References
[1] BIAN Xiu-fang, ZHANG Zhong-hua. Effect of strontium modification on hydrogen content and porosity shape of Al-Si alloys [J]. Mater Sci Forum, 2000, 331: 361-366.
[2] SHABESTARI S G, MIRESMAEILI S M, BOUTORABI A. Effect of Sr-modification and melt cleanliness on melt hydrogen absorption of 319 aluminum alloy [J]. Materials Science, 2003, 38: 1901-1907.
[3] ARGO D, GRUZLESKI J E. Porosity in modified aluminum alloy casting[J]. AFS Trans, 1988, 16: 65-74.
[4] MAO Xie-min, OUYANG Zhi-ying, ZHANG Jin-rong. A low environmental load modifying and refining treatment of casting Al alloys with RE[J]. Materials Science Forum, 2005, 475: 429-432.
[5] ZHAO Yu-guang, LI Dao-yun. Fixation hydrogen by rare earth in Al-Si alloy [J]. Acta Metallurgica Sinica, 1993, 29: A16-A19. (in Chinese)
[6] NI Hong-jun, SUN Bao-dem, JIANG Hai-yan, DING Wen-jiang. Effect of new flux including rare on A356 alloy[J]. The Chinese Journal of Nonferrous Metals, 2001, 11: 547-552. (in Chinese)
[7] YU Yun, SHU Da, XU Zheng-ming, LI Jian-guo. Effects of hydrogen in lanthanum on hydrogen content in Al-alloy[J]. Mater Sci Eng A, 2003, A357: 406-411.
[8] HAN Kui, MAO Xie-min, OUYANG Zhi-ying, MIAO Chuang-rong, ZHANG Guang-quan. Rare earth behavior in cast aluminum alloy melt during the course of degassing[J]. Special Casting & Nonferrous Alloys, 2004, 2: 15-16. (in Chinese)
[9] LIU L, SAMUEL A M, SAMUEL F H. Influence of oxides on porosity formation in Sr-treated Al-Si casting alloys[J]. Journal of Materials Science, 2003, 38: 1255-1267.
[10] YU Yun, JI Cheng-chang, LI Jian-guo, SHANG Bao-lu. The mechanism of Lanthanum dehydrogenation on aluminum alloy [J]. Foundry Technology, 2002, 6: 390-392.
[11] LESSITER M J. Understanding inclusions in aluminum castings [J]. Modern Casting, 1993, 83: 83-90.
[12] CHEN X, ENGLERS G. Formation of gas porosity in aluminum alloys [J]. AFS Trans, 1994, 102: 673-682.
[13] ZAI Jin-kun. Metal Corrosion in High Temperature[M]. Beijing: Beijing University of Aeronautics and Astronautics Press, 1993.
[14] SUN Wei-cheng, ZHANG Shu-rong, HOU Ai-qing. Behaviors of RE Elements in Aluminum Alloys[M]. Beijing: Weapon Industry Press, 1992.
[15] YANG Hui. The Structure and Property Relationship in Rare Earth Aluminum Alloy Surface Oxide Film[D]. Beijing: North China University of Technology, 2002.
[16] LU Shu-xun, GU Kai-dao, ZHENG Lai-shu. The Foundry and Smelting of Nonferrous Alloy[M]. Beijing: Defence Industrial Press, 1983.
(Edited by LONG Huai-zhong)
Corresponding author: OUYANG Zhi-ying; Tel: +86-21-56337342; E-mail: ouyangzsh@graduate.shu.edu.cn


