Crystallization of Mg-based bulk metallic glass
CHEN Gang(陈 刚)1, M. FERRY2
1. School of Materials Science and Engineering, Jiangsu University, Zhenjiang 212013, China;
2. School of Materials Science and Engineering, University of New South Wales, Sydney, NSW 2054, Australia
Received 13 November 2005; accepted 3 April 2006
Abstract:
Mg-based bulk metallic glass fabricated by conventional copper mould method was aged at different temperatures. X-ray diffractometry(XRD), scanning electron microscopy(SEM), atomic force microscopy(AFM) and focused ion beam(FIB) miller were employed to examine specimens obtained under different conditions. The crystallization of Mg-based bulk metallic glass depends upon both the aging temperature and the aging time. As temperature increases or the holding time increases, the microstructure of the aged specimen varies from glassy one to crystalline one plus glassy phase and then to absolutely multiphase crystalline one. From the FIB images, it is clear that Mg-based bulk metallic glass could not only crystallize completely but also display dendrite-like growth style. From the AFM images, there are not only significant variations of microstructures but also surface morphology of specimens obtained under different conditions. It is proposed that the surface morphology varies as the treating temperature increases. The Vickers hardness of different specimens increases as the fraction of crystalline phase (s) increases.
Key words:
Mg-based alloy; bulk metallic glass; crystallization; microstructure;
1 IntroductionSince INOUE et al[1,2] reported that amorphous alloy with the composition of Mg65Cu25Y10 could be produced with thickness up to 4 mm by conventional mold casting technique, Mg-based bulk metallic glasses (BMGs) have been proposed as a new kind of bulk metallic glasses due to their relatively low density[1-13]. It is known that an amorphous phase can serve as a precursor to prepare an expected structure, e.g., nanocrystalline structure. The difference in the densities between the as-cast bulk metallic glass and the fully crystallized state is in the range of 0.30%-0.54%, which is smaller than the previously reported values of about 2% for conventional amorphous alloys with much high critical cooling rate Rc>105 K/s[14]. Such difference indicates that the multi-component bulk metallic glasses have dense randomly packed structure and new atomic configuration. Therefore, it is necessary to investigate the crystallization behavior and the corresponding products for the structure design of these alloys.
LINDEROTH et al[15] have reported the thermal stability of Mg60Cu30Y10 bulk metallic glass in the supercooled liquid region, where Tg= 408 K and Tx= 458 K. The transition from an amorphous to fully crystalline state was studied by X-ray diffractometry(XRD) as a function of time at specific temperatures in the above region. These work subsequently generated a temperature-time-transition(TTT) phase diagram, which was used as the basis for selecting the optimum temperature in the supercooled liquid region for carrying out deformation/shaping operations on their Mg-based alloys. Despite this work, little research on the crystallization behavior of Mg-Cu-Y amorphous system has been reported. The aim of the present work is to study the crystallization behavior of the Mg65Cu25Y10 bulk metallic glass under different aging conditions. The Vickers hardness(HV) of different specimens was also examined to learn the variation of the mechanical property as the phase contents change.
2 ExperimentalMg-based alloy was produced in ingot form by melting high purity magnesium (99.98%) and a Cu-Y master alloy in an electrical resistance furnace under argon atmosphere. The Cu-Y master alloy was prepared by arc melting high purity copper and yttrium (99.99%) in an argon atmosphere. Mg65Cu25Y10 bulk metallic glass with a thickness of 2 mm was then prepared by the traditional copper mold method[15]. The amorphous phase was examined by standard X-ray diffraction. Differential scanning calorimetry(DSC) analysis with a heating rate of 20 K/min in Fig.1 shows that Tg and Tx are 418 and 477 K, respectively. The glassy specimens were cut into small pieces of 5 mm×5 mm×2 mm and reheated to expected temperatures and then held for different times. The as-cast glassy specimen and the specimens obtained under different aging conditions were then examined under scanning electron microscope (SEM), focused ion beam(FIB) miller, and atomic forced microscope(AFM).
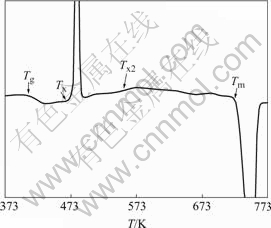
Fig.1 Differential scanning calorimetry analysis result of Mg65Cu25Y10 bulk metallic glass with a heating rate of 20 K/min
A Philip X1400 X-ray diffractometer was used to examine the phase constituents of the specimens under various stages of heat treatment. In order to eliminate the probable influence of surface oxidation, all the specimens were mechanically polished before XRD analysis. After mechanical polishing, microstructures of these specimens were then identified under SEM using a Hitachi S4500 FEGSEM. Seiko 8800 FIB miller was employed to further examine these specimens. The surfaces of different specimens were studied by using a Dimension-3000 AFM machine. AFM examination was carried out in contact imaging mode in the air and a V-shaped silicon nitride cantilever with a standard pyramidal tip was used.
The Vickers hardness testing was carried out by a HVS-50 machine. The hardness was determined by indenting each specimen 20 times under a load of 500 N for 5 s at room temperature and measuring the indentation sizes by optical microscopy.
3 Results and discussionFig.2 shows the X-ray diffraction patterns of different specimens. The specimens shown in Figs.2(a), (b), (c), and (d) are the as-cast glass, the ones aged at 463, 483 and 503 K for 9 min, respectively. There is only a halo in the pattern in Fig.2(a), while the halo is much smaller and deformed in Fig.2(b). In Fig.2(c), there are two crystalline peaks in the pattern, which are identified as Mg2Cu phase[17]. Meanwhile, there are more crystalline peaks, which are identified as magnesium solid solution, Mg2Cu, Cu2Y, and Mg24Y5, in Fig.2(d). From this figure, it is clear that the Mg-Cu-Y alloy changes from absolute glassy phase to modified glassy phase (probably containing an icosahedral phase, as K?STER et al[18] reported), Mg2Cu phase plus glassy one, and then to absolute crystalline phases as the aging temperature increases. Further study shows that there is similar tendency of the microstructure as the holding time increases from 3 to 9 min at 503 K.

Fig.2 X-ray diffraction patterns for as-cast amorphous specimen (a) and specimens aged at 463 K (b), 483 K (c), and 503 K (d) for 9 min
Fig.3 shows the SEM images of different specimens. The SEM image of the as-cast amorphous specimen is given in Fig.3(a). It can be seen that there is gray amorphous matrix except a few white oxide particles. For the specimen aged at 463 K for 9 min, which is shown in Fig.3(b), there are small white particles with a diameter of about several hundred nanometers in the gray matrix, which is similar to the matrix in Fig.3(a). Fig.3(c) shows the one aged at 483 K for 9 min. It can be seen that there are white particles with diameter varying from several hundred nanometers to one micrometer surrounded by gray layers. Fig.3(d) shows the one aged at 503 K for 9 min. The white particles in this specimen are about 2 μm in diameter, embedded in the gray matrix rather than surrounded by gray layers shown in Fig.3(c). Therefore, it is clear to see that the microstructure of the alloy varies with the aging temperature, which agrees well with the XRD results.
Images of different specimens obtained under an FIB miller are shown in Fig.4. Fig.4(a) shows the one aged at 463 K for 9 min, and Fig.4(b) shows the one aged at 503 K for 9 min. According to Figs.4 and 3, it is clear that the shape of white particle shown in Fig.3 varies with aging temperature. As the aging temperature is 463 K, the white particles look like flowers with small petals. As the temperature is 483 K, there are similar particles or flowers with small petals except with larger size, which are not shown here. Dendrites rather than particles or flowers appear in the image of the one aged at 503 K. This result agrees well with the X-ray diffraction pattern for the specimen aged at 503 K for 9 min, which shows several different phases. The FIB miller images give more details of the microstructure.
We argue here that there are different growth styles at different aging temperatures. As the temperature is lower than Tx (477 K), the crystalline phase could only grow with a relatively low growth rate and finally form small particles. As the temperature is only a bit higher than Tx, i.e., 483 K, the crystalline phase can grow larger but still be in the glassy matrix. However, the crystalline phase(s) will grow rapidly as the temperature is a bit higher than Tx. Crystal(s) will grow faster in some selected crystallography orientations than others. As a

Fig.3 SEM images of as-cast amorphous specimen(a), and specimens aged at 463 K (b), 483 K (c), and 503 K (d) for 9 min

Fig.4 Focused ion beam milling images of ones aged at 463 K (a) and 503 K (b) for 9 min
result, there will be dendrites rather than particles or flowers with small pellets formed at lower temperatures. JOHNSON et al[19,20] and GEBERT et al[21] have reported dendrite structures in partially crystallized specimens with glassy matrix obtained directly from liquid alloys. All these works show dendrites with much larger sizes than those shown in the current work, which could probably be due to the much higher growth rate of the crystalline phases. The growth mechanism of the dendrites from absolute glassy phase will be studied in details elsewhere.
In order to examine the surface morphologies of different specimens, AFM was also employed. AFM images are given in Fig.5. The one of the polished as-cast amorphous specimen is shown in Fig.5(a). The as-aged ones obtained at 483 K and 503 K for 9 min are given in Figs.5(b) and (c), respectively. From this figure, it can be seen that there are larger particles formed on the specimen surface as the aging temperature increases, which agrees well with the SEM results and the FIB ones. Considering the work on precision forming of BMGs for the production of nano-devices[22], it is very important to understand the surface variation of specimen during aging. The mechanism of the surface morphology has been further discussed.
The Vickers hardness of different specimens is listed in Table 1. The hardness of the as-cast glass is almost the same as that reported in Ref.[1]. It can be seen that the hardness increases with increasing aging temperature. It should be pointed out that the volume fraction of crystalline phase increases with increasing aging temperature. This result agrees well with the work of WOLF et al[23], even though those crystalline phases mentioned there are nano-scaled.
Table 1 Vickers hardness of different specimens

1) As the aging temperature increases or the holding time increases, the microstructure of the aged specimen varies from glassy one to crystalline one plus glassy phase and then to almost absolutely crystalline one.
2) An examination under FIB miller gives that the shape of white phase varies with the aging temperature. Dendrites or flowers rather than particles appear in the image of the specimen aged at 503 K for 9 min.
3) There is also variation of surface morphology as the aging temperature varies. The higher the aging temperature, the larger the particles formed on the specimen surface.
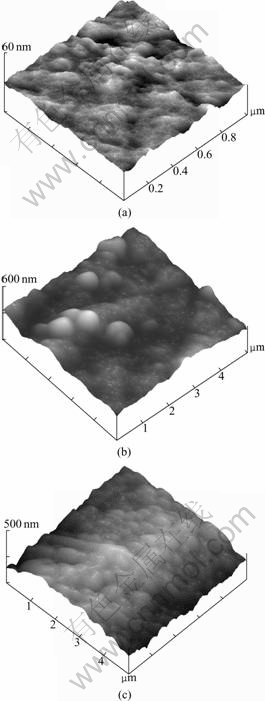
Fig.5 AFM images of polished as-cast amorphous specimen (a), and as-aged ones obtained at 483 K (b), and 503 K (c) for 9 min
4) The Vickers hardness varies with the aging condition. The volume fraction of crystalline phase and in turn the hardness will increase as the aging temperature increases.
AcknowledgementsOne of the authors, G. Chen, would like to acknowledge the School of Materials Science and Engineering at UNSW for the research facilities and the China Scholarship Council for the financial support. We also gratefully thank doctoral students Bülent Gun and Kevin Laws for specimen preparation.
References
[1] KIM S G, INOUE A, MASUMOTO T. High mechanical strengths of Mg-Ni-Y and Mg-Cu-Y amorphous alloys with significant supercooled liquid region [J]. Mater Trans JIM, 1990, 31(11): 929-934.
[2] INOUE A, KATO A, ZHANG T. Mg-Cu-Y amorphous alloys with high mechanical strengths produced by a metallic mold casting method [J]. Mater Trans JIM, 1991, 32(7): 609-616.
[3] LIU W, JOHNSON W L. Precipitation of bcc nanocrystals in bulk Mg-Cu-Y amorphous alloys [J]. J Mater Res, 1996, 11(9): 2388-2392.
[4] OHNUMA M, LINDEROTH S, PRYDS N. On the effect of Al on the formation of amorphous Mg-Al-Cu-Y alloys [J]. Mater Res Soc Symp Proc, 1999, 554: 119-124.
[5] OHNUMA M, PRYDS N, LINDEROTH S, ELDRUP M, PEDERSEN A S, PEDERSEN J S. Bulk amorpous (Mg0.98Al0.02)60Cu30Y10 alloy [J]. Scripta Metall, 1999, 41(8): 889-893.
[6] ELDRUP M, PEDERSEN A S, OHNUMA M. Bulk amorphous alloys: Preparation and properties of (Mg0.98Al0.02)x(Cu0.75Y0.25)100-x [J]. Materials Science Forum, 2000, 343(I): 123-128.
[7] KANG H G, PARK E S, KIM W T, KIM D H, CHO H K. Fabrication of bulk Mg-Cu-Ag-Y glassy alloy by squeeze casting [J]. Mater Trans JIM, 2000, 41(7): 846-849.
[8] PRYDS N H, ELDRUP M, OHNUMA M. Preparation and properties of Mg-Cu-Y-Al bulk amorphous alloys [J]. Mater Trans JIM, 2000, 41(11): 1435-1442.
[9] PARK E S, KANG H-G, KIM W T. The effect of Ag on the glass forming ability and crystallization in Mg-Cu-Ag-Y alloys [J]. Materials Science Forum, 2001, 360-362: 95-100.
[10] LINDEROTH S, PRYDS N H, OHNUMA M. On the stability and crystallization of amorphous Mg-Cu-Y-Al alloy [J]. Mater Sci & Eng A, 2001, 304-306: 656-659.
[11] PARK E S, HANG H G, KIM W T. The effect of Ag addition on the glass-forming ability of Mg-Cu-Y metallic glass alloys [J]. J Non-Cryst Soli, 2001, 279(2-3): 154-160.
[12] MEN H, HU Z Q, XU J. Bulk metallic glass formation in the Mg-Cu-Zn-Y system [J]. Scripta Mater, 2002, 46(10): 699-703.
[13] MA H, MA E, XU J. A new Mg65Cu7.5Ni7.5Zn5Ag5Y10 bulk metallic glass with strong glass-forming ability [J]. J Mater Res, 2003, 18(10): 2288-2291.
[14] INOUE A, NEGISHI T, KIMURA H M. High packing density of Zr- and Pd-based bulk amorphous alloys [J]. Materials Transactions, JIM, 1998, 39(2): 318-321.
[15] LINDEROTH S, PRYDS N, ELDRUP M. Bulk Mg-Cu-Y-Al alloys in the amorphous, supercooled liquid and crystalline states [J]. Materials Research Society Symposium Proceedings, 2001, 644(L4.1): 1-12.
[16] INOUE A. Stabilization of metallic supercooled liquid and bulk amorphous alloys [J]. Acta Mater, 2000, 48(1): 279-306.
[17] GINGL F, SELVAM P, YVON K. Structure refinement of Mg2Cu and a comparison of the Mg2Cu, Mg2Ni and Al2Cu structure types [J]. Acta Cryst B, 1993, B49(II): 201-203.
[18] K?STER U, ZANDER D, JAINLEWING R. Crystallization in Zr-Cu-Ni-Al metallic glasses [J]. Materials Research Society Symposium Proceedings, 2001, 644(L5.8): 1-6.
[19] HAYS C C, KIM C P, JOHNSON W L. Improved mechanical behavior of bulk metallic glasses containing in situ formed ductile phase dendrite dispersions [J]. Mater Sci & Eng, 2001, A304-306: 650-655.
[20] SZUECS F, KIM C P, JOHNSON W L. Mechanical properties of Zr56.2Ti13.8Nb5.0Cu6.9Ni5.6Be12.5 ductile phase reinforced bulk metallic glasses composite [J]. Acta Mater, 2001, 49(9): 1507-1513.
[21] ECKERT J, KUHN U, MATTERN N, HE G, GEBERT A. Structural bulk metallic glasses with different length-scale of constituent phases [J]. Intermetallics, 2002, 10(11-12): 1183-1190.
[22] SAOTOME Y, ITOH K, ZHANG T. Superplastic nanoforming of Pd-based amorphous alloy [J]. Scripta Mater, 2001, 44 (8-9): 1541-1545.
[23] WOLF U, PRYDS N, JOHNSON E, WERT J A. The effect of partial crystallization on elevated temperature flow stress and room temperature hardness of a bulk amorphous Mg60Cu30Y10 alloy [J]. Acta Materialia, 2004, 52(7): 1989-1995.
(Edited LONG Huai-zhong)
Foundation item: Project(2005) supported by the Science Foundation of the Education Administration for Returning Oversea Scholars; Project (2004JDG018) supported by the Natural Science Foundation of Jiangsu University, China
Corresponding author: CHEN Gang; Tel: +86-511-8791276; Fax: +86-511-8791919; E-mail: gchen@ujs.edu.cn



