
Effect of current density on corrosion resistance of micro-arc oxide coatings on magnesium alloy
YANG Yue(杨 悦), WU Hua(吴 化)
Key Laboratory of Advanced Structural Materials, Ministry of Education, Changchun University of Technology, Changchun 130012, China
Received 23 September 2009; accepted 30 January 2010
Abstract:
Oxide coatings were prepared on magnesium alloys in electrolyte solution of Na2SiO3 at different current densities (3, 4 and 5 A/cm2) with micro-arc oxidation process. X-ray diffractometry (XRD) results show that the oxide coatings formed on magnesium alloys are mainly composed of MgO and MgAl2O4 phases; in addition, the content of MgO increases with increasing the current density. The morphology and surface roughness of the coatings were characterized by confocal laser scanning microscopy (CLSM). The results show that the surface roughness (Ra) decreases with increasing the current density. Moreover, the electrochemical corrosion results prove that the MgO coating produced in the electrolyte Na2SiO3 at current density of 5 A/cm2 shows the best corrosion resistance.
Key words:
magnesium alloy; micro-arc oxidation; current density; corrosion resistance;
1 Introduction
Magnesium alloys are attractive for applications in the automobile and aerospace industries because of their relatively low density and high damping capacity as compared to other structural metals[1-4]. However, magnesium has a number of undesirable properties including poor corrosion and wear resistance, poor creep resistance and high chemical reactivity, which have limited their more extensive use in many applications. Techniques for solving these problems include electroplating, chemical plating, anodic oxide coating, chemical conversion coating, physical vapor deposition, surface coating, laser surface treatment and so on, but all of these methodologies have specific limitations[5-7].
Micro-arc oxidation (MAO) is a relatively new and promising surface treatment method used to form a thick oxide coatings on magnesium substrate by plasma discharge in aqueous solution on substrate surface under high voltage. The deposition process of MAO implies electrochemical, plasma chemical and thermal chemical reactions taking place in the electrolyte[8-10]. The quality of MAO coating can be controlled by compositions of electrolyte, temperature of electrolyte, treatment time and voltage, anodic current density, etc [11-15]. Generally, current density is one of the most important parameters affecting microstructure and properties of the coating. However, there is little research about the effects of current density on the oxide coatings on magnesium alloys. In this work, the effect of current density on the corrosion resistance of the coatings formed on magnesium alloys was analyzed. It is expected that the preliminary results can be significant in promoting the further application of MAO process about magnesium alloy.
2 Experimental
2.1 Preparation of MAO coatings on magnesium alloy
Rectangular coupons (10 mm×20 mm×3 mm) of AZ91D magnesium alloy were used as substrates in the experiment. Prior to the MAO treatment, the specimens were polished with SiC abrasive paper (up to1000 grit) and degreased in acetone and distilled water. MAO units used in this work mainly consist of electrolyte bath with stirring and cooling systems and a power supply with approximate 1 000 V of the maximum voltage amplitude. The MAO process was performed in alkaline electrolyte containing 15 g/L Na2SiO3 and 3 g/L NaOH. The temperature of the electrolyte was controlled at 20-30 ?C. For the power supply, its frequency was 500 Hz. The current density varied at 3, 4 and 5 A/dm2 at the coating surface was maintained by controlling the voltage amplitude in the deposition process.
2.2 Characterization of MAO coatings
The phase composition of the coatings was identified with X-ray diffractometer (Model D/Max 2500PC Rigaku, Japan) operated with Cu Kα. The X-ray generator settings were 50 kV and 50 mA, and the scans were acquired from 20? to 80? (in 2θ). The microstructure and surface roughness of the coatings were observed by confocal laser scanning microscope (Olympus OLS3000, Japan), which uses advanced image processing software and allows the construction of three-dimensional images of the object, topographical maps, and the surface roughness values of the coatings could also be obtained.
2.3 Corrosion resistance tests
The corrosion resistances of the substrate and the coatings prepared at different current densities were evaluated by means of a three-electrode corrosion cell. 3.5% (mass fraction) NaCl solution was used as corrosive medium. A platinum electrode was used as counter electrode and a saturated calomel electrode (SCE) was used as reference. The working electrode had an area of 1 cm2 in contact with the electrolyte. The potentiodynamic polarization curves were scanned from -2.2 V to -2.0 V at a rate of 10 mV/s with a potentiostat EG&G model 273. The surface morphologies of the oxide coatings after the corrosion resistance tests were characterized by SEM.
3 Results and discussion
3.1 Effect of current density on microstructure and surface roughness of micro-arc oxidation coating
As is known there are three main steps underlying the formation of MAO coatings: firstly, a number of discharge channels are formed for the breakdown of the low conductive oxide layer and the alloy elements are melted out of the substrate to the channels; then, the alloy elements react with the electrolytic elements and get oxidized; at last, the oxidized material is ejected to the coating surface and cooled by the electrolyte. The above processes are repeated all through the MAO process, leading to the increase of the coating thickness. Therefore, the morphology and structure of the coating are quite dependent on the oxidation process. Fig.1 illustrates the two-dimensional and three-dimensional images of the coatings formed on the magnesium alloy in a bath containing 20 g/L Na2SiO3 and 3 g/L NaOH at different current densities for 40 min. The micrographs clearly indicate that the pores present on the coating surface as dark circular spots and distribute all over the surface of the ceramic coatings. It is also apparent that the number of pores is increased, while the diameter of the pores is apparently decreased with increasing the current density. In Fig.2(a), some micro cracks inevitably appear on the coating due to the rapid solidification of the molten MgO in the discharge channel. In contrast to this, more unsealed micro-pores are found to exist in the coating formed at the current densities of 4 A/dm2 and 5 A/dm2.
The corresponding three-dimensional images of the coatings are also displayed in Fig.1. Though the coatings take on the similar surface figures, the wave crest and trough of the agglomeration vary depending on the current density, which causes different surface roughness. The results of surface roughness measured with the software attached to the CLSM are presented in Table 1. It is clearly that the surface roughness decreases with increasing the current density. The change of surface morphologies can be exclusively contributed to the effect of current density, which determines the applied voltage in the MAO process. The higher the applied voltage in the process, the higher the energy for the micro-pores to sinter together. Therefore, the higher the current density, the smaller the dimension in the discharge channel, and the lower the surface roughness in the surface appearance.
Table 1 Thickness and surface roughness of MAO coatings formed at different current densities

3.2 Effect of current density on composition of micro- arc oxidation coating
Fig.2 shows X-ray diffraction patterns of oxide coatings formed on magnesium alloys by micro-arc oxidation in a bath containing 15 g/L Na2SiO3 and 3 g/L NaOH at current densities of 3, 4 and 5 A/dm2 for 40 min, respectively. It is obvious that the MAO coating consists of MgO and MgAl2O4 phases. It is known that the magnesium oxide is produced by plasma thermal chemical reactions in the discharging channels. The phase is thermodynamically stable at all testing temperatures, whereas is metastable. The magnesium oxide should be formed by plasma thermal chemical reactions in the discharge channels. When the current density is higher, the discharge spark can burn for a long time in MAO process. So, the discharge product can be transformed completely into the phase. In addition, the content of MgO in the coatings has no apparent variation
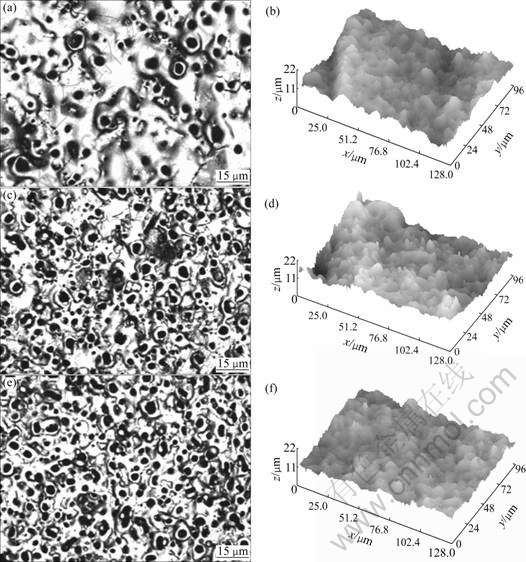
Fig.1 Surface CLSM images and corresponding three-dimensional images of ceramic coatings formed at different current densities: (a), (b) 3 A/dm2; (c), (d) 4 A/dm2; (e), (f) 5 A/dm2
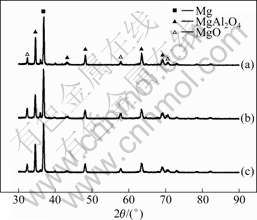
Fig.2 XRD patterns of MAO coatings formed on magnesium alloys at different current densities: (a) 3 A/dm2; (b) 4 A/dm2; (c) 5 A/dm2
with the current density. However, the content of MgAl2O4 in the coating formed at the current density of 5 A/dm2 is decreased compared to the other two coatings fabricated at current densities of 3 and 4 A/dm2, respectively. It is well known from the previous study that, the content of MgAl2O4 has a strong effect on the corrosion resistance of the MAO coating.
3.3 Effect of current density on corrosion resistance of micro-arc oxidation coating
In order to evaluate the corrosion resistance provided by ceramic coatings, potentiodynamic polarization curves were performed. The representative polarization curves for magnesium alloys coated with ceramic coatings by micro-arc oxidation and for the substrate are shown in Fig.3. It is obvious that the sample treated by micro-arc oxidation has a good corrosion protective property compared with the magnesium substrate; furthermore, the coating fabricated at current density of 5 A/dm2 shows the best corrosion resistance. This may be attributed to the morphology and roughness of the coatings discussed above. It has been reported that the main corrosion form of magnesium alloy in NaCl solution is pitting corrosion[16]. Therefore, the micro-pores of the MAO coatings have detrimental effect on the corrosion performance. Larger pores augment the real exposed area to the corrosive solution and they may concentrate more corrosion medium than little ones. The excellent corrosion resistance is possibly attributed to compact structure of the coating fabricated in electrolyte containing 20 g/L Na2SiO3 and 3 g/L NaOH at the current density of 5 A/dm2. The corresponding surface morphologies of oxide films after corrosion tests formed at different current densities are shown in Fig.4.

Fig.3 Polarization curves of substrate and ceramic coatings formed at different current densities: (a) Substrate, magnesium alloy; (b) Coating, 3 A/dm2; (c) Coating, 4 A/dm2; (d) Coating, 5 A/dm2
4 Conclusions1) The current density has a strong effect on the corrosion resistance of MAO coatings.
2) By changing the current density, the MAO coatings possess different compositions and microstructures, as well as the corrosion resistances. The oxide coatings consist mainly of MgO and MgAl2O4 phases. However, the content of MgAl2O4 in the coating formed at current density of 5 A/dm2 decreases compared to the other two coatings fabricated at current densities of 3 and 4 A/dm2.
3) As a result, the coating formed at current density of 5 A/dm2 shows the better corrosion resistance than the other MgO coatings.

Fig.4 Surface morphologies of oxide films formed at different current densities after corrosion tests: (a) 3 A/dm2; (b) 4 A/dm2; (c) 5 A/dm2
References[1] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—A critical review [J]. J Alloys Comp, 2002, 336: 88-113.
[2] DABAL? M, BRUNELLI K, NAPOLITANI E, MAGRINI M. Cerium-based chemical conversion coating on AZ63 magnesium alloy [J]. Surf Coat Tech, 2003, 172: 227-232.
[3] ZHAO M, WU S, LUO J. R, FUKUDA Y, NAKAE H. A chromium-free conversion coating of magnesium alloy by a phosphate-permanganate solution [J]. Surf Coat Tech, 2006, 200: 5407-5412.
[4] KOUISNI L, AZZI M, ZERTOUBI M, DALARD F,MAXIMOVITCH S. Phosphate coatings on magnesium alloy AM60. Part 1. Study of the formation and the growth of zinc phosphate films [J]. Surf Coat Tech, 2004, 185: 58-67.
[5] SHARMA A K, SURESH M R, BHOJRAJ H, NARAYANAMURTHY H, SAHU R P. Electroless nickel plating on magnesium alloy [J]. Met Finish, 1998, 96: 10-14.
[6] HSIAO H Y, TSAI W T. Characterization of anodic films formed on AZ91D magnesium alloy [J]. Surf Coat Tech, 2005, 190: 299-308.
[7] AMBAT R, ZHOU W. Electroless nickel-plating on AZ91D magnesium alloy: Effect of substrate microstructure and plating parameters [J]. Surf Coat Tech, 2004, 179: 124-134.
[8] BAI A, CHEN Zhi-jia. Effect of electrolyte additives on anti-corrosion ability of micro-arc oxide coatings formed on magnesium alloy AZ91D [J]. Surf Coat Tech, 2009, 203: 1956-1963.
[9] SU Pei-bo, WU Xiao-hong, GUO Yun, JIANG Zhao-hua. Effects of cathode current density on structure and corrosion resistance of plasma electrolytic oxidation coatings formed on ZK60 Mg alloy [J] J Alloys Compd, 2009, 475: 773-777.
[10] LUO Hai-he, CAI Qi-zhou, WEI Bo-kang, YU Bo, HE Jiang, LI Ding-jun. Study on the microstructure and corrosion resistance of ZrO2-containing ceramic coatings formed on magnesium alloy by plasma electrolytic oxidation [J] J Alloys Compd, 2009, 474: 551-556
[11] LEE J M, KANGA S B, HAN J M. Dry sliding wear of MAO-coated A356/20vol.% SiCp composites in the temperature range 25-180 ?C [J]. Wear, 2008, 264: 75-85.
[12] CHEN Chang-jun, WANG Mao-cai, WANG Dong-sheng, JIN Ren, LIU Yi-ming. Microstructure and corrosion behavior of Mg-Nd coatings on AZ31 magnesium alloy produced by high-energy micro-arc alloying process. [J] J Alloys Compd, 2007, 438: 321-326.
[13] CHEN Chang-jun, WANG Mao-cai, LIU Yi-ming, WANG Dong-sheng, JIN Ren. Mass transfer trends and the formation of a single deposition spot during high-energy micro-arc alloying of AZ31 Mg alloy [J] J Materials Processing Technology, 2008, 198: 275-280.
[14] WU Wei-yi, HAN Yong. Characterization and properties of the MgF2/ZrO2 composite coatings on magnesium prepared by micro-arc oxidation [J]. Surf Coat Tech, 2008, 202: 4278-4284.
[15] JANG Y S, KIM Y K, PARK I S. Film characteristics of anodic oxidized AZ91D magnesium alloy by applied power [J]. Surf Interface Anal, 2009, 41: 524-530.
[16] GUO Hong-fei, AN Mao-zhong, XU Shen, GUO Hui-bin. Microarc oxidation of corrosion resistant ceramic coating on a magnesium alloy [J]. Mater Lett, 2006, 60(12): 1538-1541.
Foundation item: Project(20080505) supported by Science and Technology Department of Jilin Province, China
Corresponding author: WU Hua; Tel: +86-431-85716426; E-mail: wuhua@mail.ccut.edu.cn


