
Biosorption behaviors of Cu2+, Zn2+, Cd2+ and mixture by waste activated sludge
LUO Sheng-lian(罗胜联)1, 2, YUAN Lin(袁 林)1, CHAI Li-yuan(柴立元)1,
MIN Xiao-bo(闵小波)1, WANG Yun-yan(王云燕)1, FANG Yan(方 艳)1, WANG Pu(王 璞)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China
Received 27 April 2006; accepted 12 September 2006
Abstract:
Biosorption of heavy metal ions, such as Cu2+, Cd2+ and Zn2+, was carried out using waste activated sludge from municipal sewage treatment plant as adsorption material, and the effects of parameters, such as pH value, temperature, reaction time and sorption duration, were studied in detail. The results indicate that the removal rates of Cu2+, Zn2+ and Cd2+ with low concentration are 96.47%, 80% and 90%, respectively, adsorbed by waste activated sludge. Little effect of dosage of activated sludge on the adsorption of Cu2+ and more effects on the adsorption of Zn2+and Cd2+ are observed. Little effect of temperature is observed, while pH value and adsorption time exert important influence on the sorption process. The adsorption behaviors of heavy metal ions all have parabolic relationships with pH value. The optimum pH value is between 6 and 10, and the optimum adsorption time is 1 h. In single heavy metal ion system, the sorption processes of Cu2+, Zn2+ and Cd2+ are in accordance with Freundlich model, which indicates that it is suitable for the treatment of these three heavy metal ions using intermittent operation. In addition, the sorption capacity of the sludge for Cu2+ is preferential to the other two ions.
Key words:
biosorption; waste activated sludge; heavy metal mixture;
1 Introduction
Waste activated sludge is the by-product of sewage treated by activated sludge process. According to incomplete statistic, in China sewage discharge amount is 4.474×107 m3/d[1], and there are more than 100 sewage treatment plants with different scales. Sludge amount accounts for 0.5%-1.0% of sewage treatment amount. Taking 100 000 t sewage per day into account, sludge amount with water-content coefficient of about 80% is 60-80 t, accordingly dry sludge amount is 10- 16 t per day. At present, waste sludge of most sewage treatment plants is landfilled simply[2,3], which produces secondary pollution easily.
More than 90% organisms mainly exist in the structure of fattiness in waste activated sludge and abundant function groups, such as —NH2, —NH, —OH, —C=O, C=C, CH3— and CH2—. So sludge is worth reusing as preparing oil, carbon, fertilizer, and so forth[4-7]. In addition, researchers also focus on treating heavy metal wastewater using activated sludge[8-10]. JIANG et al[11] studied the adsorption of Cu2+ and Zn2+ by surplus activated sludge in reducing sewage sludge metal concentration, and the optimum conditions were obtained: pH=7.0, t=1 h, addition ratio=2.0 g/L. WU et al [12,13] studied the biosorption of heavy metal mixture by sludge, and built the corresponding adsorption model. BUX et al[14], ATKISON et al[15] studied the adsorption of zinc contained wastewater with high concentration (more than 100 mg/L) by activated sludge, and the results indicated that the removal rate reached up to 96%. However, there is little report on treating heavy metal wastewater with low concentration by waste activated sludge.
A small amount of heavy metal ions in water body came from discharged water after conventional treatment will cause biological magnification, so advanced treatment should be applied. In this study, waste treatment of heavy metals mixture with low concentration (the concentrations of Cu2+, Zn2+ and Cd2+ are all lower than 50 mg/L) by waste activated sludge is studied. The effects of dosage of sludge, reaction time, temperature, and pH value on biosorption of heavy metal are investigated in detail, and then sorption isotherms of Cu2+, Zn2+ and Cd2+ are obtained, which provide foundations for exploiting sludge as a water treating agent.
2 Experimental
2.1 Materials
Waste activated sludge was sampled from the oxidation ditch of Changsha Sewage Treatment Plant, and the water-content coefficient was 74.5%. It was preserved in refrigerator.
Wastewater containing heavy metal ions Zn2+ 8.5 mg/L, Cu2+ 8.5 mg/L, Cd2+ 5 mg/L were confected with chloride (AR) and pure water.
2.2 Experimental procedure
The waste sludge with certain volumes was put into conical flasks and 100 mL waste confected water was added. After reaction for certain time the mixture was placed in shaking bed under shaking rate of 125 r/min. Then up-clear solution was taken and filtrated, and the concentrations of heavy metal ions were analyzed by atom absorption spectrophotometer[16].
3 Results and discussion
3.1 Effect of dosage of activated sludge on biosorption rate of heavy metals
Effects of dosage of activated sludge on biosorption rate of heavy metals are shown in Fig.1. From Fig.1 it can be seen that the sequence of removal rate of several heavy metals by activated sludge with same dosage is Cu2+>Cd2+>Zn2+. The removal rates of Cu2+, Zn2+ and Cd2+ reach up to 94.12%, 75.29%, and 82% respectively when adding 1.0 g wet sludge. Accordingly, the concentrations of heavy metals of treated water are 0.5 mg/L, 2.1 mg/L and 0.9 mg/L, respectively. When dosage of sludge is increased to 6.0 g, the removal rates of Cu2+, Zn2+ and Cd2+ are 96.47%, 89.41% and 98%. This indicates that removal rates of Zn2+, Cd2+ are enhanced with the dosage of sludge increasing, but it has little effect on removal of Cu2+. Therefore the optimum dosage of activated sludge is 3.0 g, the removal rates of Cu2+, Zn2+ and Cd2+ are up to 96.47%, 80% and 90%, and the biosorption contents per unit are 0.27, 0.23 and 0.15 mg/g, respectively.
3.2 Effect of reaction time on biosorption of heavy metal ions by sludge
Fig.2 shows the effect of reaction time on biosorption of heavy metal ions by activated sludge. For Cu2+, biosorption rate reaches up to 90% when reaction time is 0.5 h, and then little change of biosorption rate is observed. The biosorption rate is 96.47% when reacting for 7 h. This illustrates that there is little effect of reaction time on removal rate of Cu2+ biosorpted by waste activated sludge. However, for Zn2+ and Cd2+, biosorption rates are 47.06% and 58% when reaction time is 0.5 h, and then biosorption rate rises step by step with reaction time prolonging; and biosorption rates are up to 89.41%, 96% when reaction time is 7 h. Therefore, it is obvious that waste activated sludge shows preferential biosorption of Cu2+.
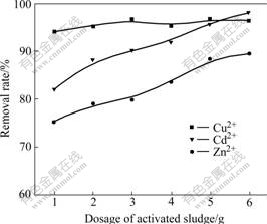
Fig.1 Effect of dosage of activated sludge on biosorption rate of heavy metal ions
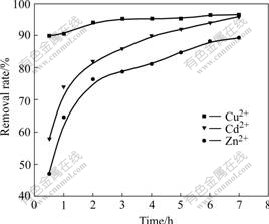
Fig.2 Effect of reaction time on biosorption rate of heavy metal ions by activated sludge (25 ℃, pH=5.33)
3.3 Effect of temperature on biosorption rate of heavy metal ions by sludge
Effects of temperature on biosorption rate of heavy metal ions by sludge are shown in Fig.3.
Biosorption rate of Cu2+ maintains at about 94% when temperature changes from 10 ℃ to 45 ℃; biosorption rate of Zn2+ rises from 58.82% up to 70.51%; and biosorption rate of Cd2+ rises from 80% to 88%. It could be concluded that biosorption rates of Zn2+, Cd2+ are promoted to a little extent with temperature increasing. So biosorption could be conducted under room temperature.
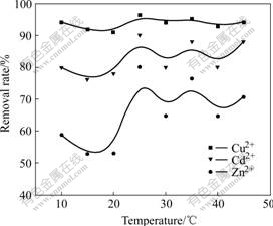
Fig.3 Effect of temperature on biosorption rate of heavy metal ions by sludge (pH=5.33, 1 h)
3.4 Effect of pH value on biosorption rate of heavy metal ions by sludge
Fig.4 represents the effect of pH value on biosorption rate of heavy metal ions by sludge. From Fig.4 it can be seen that there is an obvious effect of pH value on biosorption of heavy metal ions by activated sludge. The relationships between pH value and biosorption rate of Cu2+, Zn2+ and Cd2+ are all parabolic, which indicates that there exists an optimum range of pH value. In strong acidic solution, H+ competes for active biosorption site with heavy metal ions, which impedes biosorption of heavy metal ions by sludge, and biosorption amount of heavy metal ions decreases with acidity increasing. While in alkaline solution, hydrates formed with hydrolyzation of heavy metal ions precipitate on the surface of sludge, and can decrease the biosorption activity. Biosorption rates of Cu2+, Zn2+ and Cd2+ are all higher when pH value varies within the range of 4-10.
Fig.5 shows the effect of initial pH value on termi-nal pH value. The curve in Fig.5 could be regarded as three segments. For the first segment, terminal pH value is an up-parabola when initial pH value changes from 1 to 5; for the second segment, terminal pH value is a horizontal line maintaining about 6.5 when initial pH value varies from 6 to 10; and for the third one, terminal pH value changes largely compared with initial pH value when initial pH value ranges from 11 to 14. Therefore, the optimum value for controlling initial pH value is within 6-10.
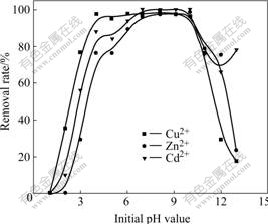
Fig.4 Effect of pH value on biosorption rate of heavy metal ions by sludge (25 ℃, 1 h)
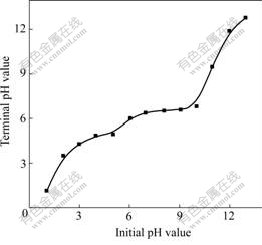
Fig.5 Effect of initial pH value on terminal pH value (25 ℃, 1 h)
3.5 Biosorption behaviors of heavy metal ion by sludge in single metal ion system
Relationship between lgq and lgce in the system containing single heavy metal ion fitted by Freundlich model is shown in Fig.6, where q is biosorption amount, c is equilibrium concentration of adsorbate. The sorption isotherm of Cu2+, Zn2+ and Cd2+ is shown in Fig.7.
Freundlich equation is lgq=lgK+1/n×lgc, where K and n are reaction constants. K could be calculated from intercept and 1/n could be calculated from slope. The Freundlich fitted results indicate that correlation efficient R of Cu2+, Zn2+ and Cd2+ biosorpted by activated sludge are 0.995 7, 0.970 9 and 0.993 2, 1/n values are 0.421 5, 0.462 4 and 0.397 8 and K values are 0.270 5, 0.200 0 and 0.374 7, respectively. Biosorption of Cu2+, Zn2+ and Cd2+ tends to be chemical adsorption judged by the values of K and 1/n, which illustrates that manipulation could be carried out intermittently for treating waste water containing single heavy metal ion (when 1/n=0.1-0.5, intermittent manipulation; while when 1/n>2, continuous manipulation).
Fig.7 indicates that biosorption amount of Cu2+ increases with equilibrium concentration of adsorbate, while that of Zn2+ maintains invariable when it reaches to a certain value.
The Langmuir isotherm for Cu2+, Zn2+, Cd2+ is shown in Fig.8. From Fig.8 and Fig.6 the Freundlich model fits better for Cu2+ with correlation coefficient R2=0.995 7 than Langmuir model with R2=0.968 28. Langmuir model fits better for Zn2+ with correlation coefficient R2=0.973 98 than Freundlich model with R2= 0.970 9. Freundlich model fits better for Cd2+ with correlation coefficient R2=0.993 2 than Langmuir model with R2=0.977 42.
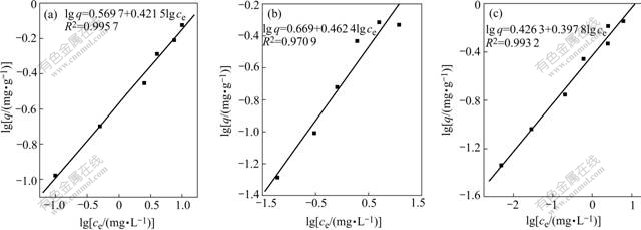
Fig.6 Relationship between lgq and lgce of Cu2+(a), Zn2+(b) and Cd2+(c)
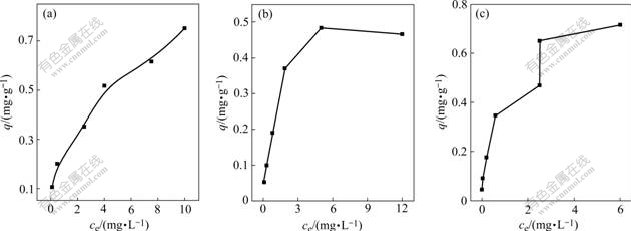
Fig.7 Sorption isotherm of Cu2+(a), Zn2+(b) and Cd2+(c)

Fig.8 Relationship between ce/q and ce of Cu2+(a), Zn2+(b) and Cd2+(c)
4 Conclusions
1) The removal rates of Cu2+, Zn2+ and Cd2+ with low concentration are 96.47%, 80% and 90% respectively using waste activated sludge.
2) Little effect of dosage of activated sludge on the adsorption of Cu2+ and more effects on the adsorption of Zn2+and Cd2+ are observed. Little effect of temperature is observed, while pH value and adsorption time exert important influence on the sorption process. The adsorption behaviors of heavy metal ions all have parabolic relationships with pH value. The optimum pH value is between 6 and 10, and the optimum adsorption time is 1 h.
3) In single heavy metal ion system, the sorption process of Cu2+, Zn2+ and Cd2+ is in accordance with Freundlich model, which indicates that it is suitable for the treatment of these three heavy metal ions using intermittent operation. The Freundlich model fits better for Cu2+, Cd2+ than Langmuir model, the model for Zn2+ is on the contrast.
4) The sorption capacity of the sludge for Cu2+ is preferential to other two ions.
References[1] NIU Ying, CHEN Ji-hua. New developments of residual sludge treatment technology [J]. Industrial Water and Wastewater, 2000, 31(5): 4-6. (in Chinese)
[2] HE Yan, ZHOU Gong-ming. Research progress on excess sludge reduction technologies [J]. Environmental Technology, 2004(1): 39-42. (in Chinese)
[3] WEI Yuan-song, PAN Yao-bo. Research and application of sludge reduction technology [J]. China Water and Wastewater, 2001, 17(7): 23-26. (in Chinese)
[4] ZHANG Li-feng, LU Rong-hu. Thermochemical process for treating residual activated sludge [J]. Environmental Protection of Chemical Industry, 2003, 23(3): 146-149. (in Chinese)
[5] XIA Zhao-hui, ZHANG Hui-li, WU Zhen. Discussion on utilization of waste activated sludge [J]. Henan Chemical Industry, 2005, 22(1): 44-45. (in Chinese)
[6] JIANG Zhan-peng. Environmental Engineering [M]. Beijing: Higher Education Press, 1992. 170-175. (in Chinese)
[7] WU Zhi-ping, YANG Gong-ping, GAO Dan. Discussion on reuse of residual sludge [J]. Shanxi Energy and Conservation, 2004(4): 33-34. (in Chinese)
[8] FUKUSHI K, CHANG D, GHOSH S. Enhanced heavy metal uptake by activated sludge cultures grown in the presence of biopolymer stimulations [J]. Wat Sei Tech, 1996, 34(5/6): 267-272.
[9] ARICAN B, GOKAY C F, YETIS U. Mechanistics of nickel sorption by activated sludge [J]. Process Biochemistry, 2002, 37: 1307-1315.
[10] LIU Hui, CHAI Li-yuan, MIN Xiao-bo, WANG Yun-yan, YU Xia. Study and development of activated sludge treatment of heavy metal containing wastewater [J]. Industrial Water and Wastewater, 2004, 35(4): 9-11. (in Chinese)
[11] JIANG Cheng-ai, WU Qi-tang, WU Shun-Hui, LIN Yi. Study on adsorption of Cu2+ and Zn2+ with waste activated sludges [J]. Journal of South China Agriculture University, 1999, 20(3): 94-98. (in Chinese)
[12] WU Qi-tang, JIANG Chang-ai, LIN Yi, LAO Wei-xiong. Application of biosorption of heavy metals by surplus activated sludge in reducing sewage sludge metal concentration [J]. Acta Scientiae Circumstantiae, 2000, 20(5): 651-653.
[13] WU Hai-suo, ZHANG Hong, ZHANG Ai-qian, WANG Lin-sheng. Biosorption of heavy metal mixture by activated sludge [J]. Acta Scientiae Circumstantiae, 2002, 21(6): 528-532.
[14] BUX F, ATKINSON B, KASAN H C. Zinc biosortion by waste activated and digested sludges [J]. Wat Sci Tech, 1999, 39(10): 127-130.
[15] ATKISON B W, BUX F, KASAN H C. Bioremediation of metal-contaminated industrial effluents using waste sludges [J]. Water Science Technology, 1996, 34(9): 9-15.
[16] State Environmental Protection Bureau of China. Measurement Method for Water and Wastewater [M]. Beijing: The National Environmental Science Press of China, 1998. 120-122.
(Edited by YANG Bing)
Foundation item: Project(50508044) supported by the National Natural Science Foundation of China; Project(05SK1003-1) supported by Key Project of Science and Technology Plan of Huana Province, China
Corresponding author: CHAI Li-yuan; Tel: +86-731-8836921; E-mail: lychai@mail.csu.edu.cn


