
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1849-1854
Preparation and hydrogen storage properties of ultrafine pure Mg and Mg-Ti particles
Stéphane PHETSINORATH1, ZOU Jian-xin1,2, ZENG Xiao-qin1,2, SUN Hai-quan1, DING Wen-jiang1,2
1. National Engineering Research Center of Light Alloy Net Forming, Shanghai Engineering Research Center of
Mg Materials and Applications, Shanghai Jiao Tong University, Shanghai 200240, China;
2. State Key Laboratory of Metal Matrix Composite, School of Materials Science and Engineering,
Shanghai Jiao Tong University, Shanghai 200240, China
Received 10 November 2011; accepted 18 June 2012
Abstract:
The ultrafine pure Mg and Mg-Ti particles were prepared through a direct current (DC) arc plasma method. The X-ray diffraction (XRD), transmission electron microscopy (TEM), pressure-composition-temperature (PCT) method and TG/DTA techniques were used to study the phase components, microstructure and hydrogen sorption properties of the powders before and after hydrogen absorption. It is revealed that most of the ultrafine Mg and Mg-Ti particles are hexagonal in shape with particle size in the range of 50-700 nm. According to the Van’t Hoff equation, the hydrogenation enthalpy of Mg-Ti powders is determined to be about -67 kJ/mol H2 based on the PCT curves of hydrogen absorption plateau pressures. This value is much higher than -78.6 kJ/mol H2 for pure Mg powders. TG/DTA analyses show that the onset dehydriding temperature of hydrogenated Mg-Ti powders is 386 ℃, which is significantly lower than that of the hydrogenated Mg (423 ℃). The results prove that the addition of Ti into Mg through arc evaporation method can improve the thermodynamic properties of Mg for hydrogen storage.
Key words:
Mg; Mg-Ti; DC arc plasma; ultrafine particles; hydrogen storage;
1 Introduction
Hydrogen is regarded as the optimist energy source as it is clean and inexhaustible. Nowadays, the challenge for hydrogen technology is the lack of safe and efficient hydrogen carriers, especially for onboard applications. In the past few decades, different kinds of hydrogen storage materials have been developed, such as metal hydrides, complex hydrides, carbon nanotubes and metal organic frameworks (MOF) [1]. Among those metal hydrides, one of the most promising candidates is MgH2[2] owing to the advantages of Mg over other metals, such as low cost, abundant resources, environment friendly nature and high hydrogen storage capacity (7.6% [3]). Moreover, Mg hydride offers a low reactivity toward air and makes it a very safe energy carrier candidate for mobile applications. Indeed, Mg hydride meets the minimum requirements described by the US Department of energy, e.g. 6.5% of gravimetric capacity and 62 kg/m3 H2 of volumetric capacity for onboard applications. However, there are still many barriers which must be overcome before further practical applications of Mg- based hydrogen storage materials, such as the slow hydrogen sorption kinetics and the high operation temperature.
Many methods have been developed to improve the hydrogen storage properties of Mg. For example, through mechanical alloying, Mg-based alloys and composites with nanostructures [4] could be produced, providing with superior hydrogen storage properties, such as fast diffusion rate and hydrogen sorption kinetics. For pure Mg, the hydrogenation enthalpy is around -74.7 kJ/mol H2 [5] and its activation energy is evaluated to be 86 kJ/mol H2 [6]. Both have been successfully reduced by addition of catalysts, including metals, chlorides, oxides, etc [7]. For example, niobium oxide is one of the most efficient catalysts, which can improve significantly the hydrogen sorption properties of Mg. It is revealed that niobium oxide plays a role as the “hydrogen pump” during hydrogen sorption, enhancing the hydrogen uptake and the diffusion of hydrogen into the Mg crystals. This mechanism can considerably bring down the absorption energy of activation, such as, 61 kJ/mol H2 at 0.2% (mole fraction) Nb2O5 [8] and even 51 kJ/mol H2 at 2%-5% (mole fraction) Nb2O5 [9]. Among those metallic elements, Ti is found to be a good catalyst element for hydrogen storage of Mg. Both theoretical and experimental investigations have shown that the hydrogen sorption thermodynamics and kinetics can be significantly improved by partial substitution of Mg by Ti in MgH2. Normally, catalyst elements or additives are introduced into Mg through mechanical milling method [6,8]. Recently, an arc plasma evaporation method is developed as an efficient way to produce ultrafine Mg particles [10]. Through a two-step method, arc evaporation followed by mechanical milling, Mg-based hydrogen storage alloys or composites can be prepared with superior properties. However, the direct preparation of Mg alloys or compounds by arc plasma method has not been studied in details yet.
Considering the above mentioned, pure Mg and Mg-Ti ultrafine powders were prepared through an arc evaporation method in the present work. The aim of this work is to study the possibility of producing Mg-based alloys directly through vapor state alloying and to investigate the effect of addition of Ti into Mg on the hydrogen sorption properties of Mg.
2 Experimental
The ultrafine pure Mg and Mg-Ti powders were prepared using an arc plasma evaporation apparatus. Figure 1 shows a schematic illustration of the experimental equipment. It mainly consists of a reaction chamber and a collecting room. Pure Mg powders were prepared by arc evaporation of bulk pure Mg with a purity of 99.9%. Ultrafine Mg-Ti particles were prepared by arc evaporation of the mixture of Mg and Ti powders. Commercially available Mg and Ti powders with 99.9% in purity and 100 μm in particle size were delicately mixed in a molar ratio of 1:2. The mixed powders were then compressed at room temperature to form cylinders with 10 μm in diameter and 7 mm in height by a uniaxial compressor under a pressure of 25 MPa. These cylinders, as anode materials, were put into the reaction chamber filled with mixed 75 kPa Ar+ 5 kPa H2 gas after the chamber was evacuated to 5×10-2 Pa. The direct current was set at 140 A during arc evaporation. Before the ultrafine Mg-Ti particles were taken out from the reaction chamber, they were slowly passivated with a mixture of argon and air to prevent the particles from burning in air.
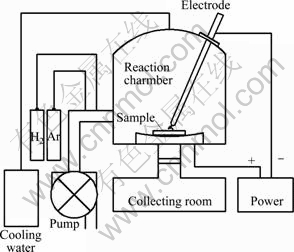
Fig. 1 Schematic illustration of DC arc plasma evaporation equipment
The phase identification of the ultrafine pure Mg and Mg-Ti particles before and after hydrogen absorption was carried out by X-ray diffraction (XRD) using a D/max 2550VL/PCX apparatus equipped with Cu Kα radiation source. The morphology and microstructure of the powders were observed by using a JEM-2100 transmission electron microscope (TEM), operated at 200 kV. The composition of the Mg-Ti powders was analyzed by inductive coupled plasma emission spectrometer (ICP). A conventional Sievert type pressure-composition-temperature (PCT) apparatus, which means measuring hydrogen content versus temperatures by recording the change of gas pressure in a constant volume, was used to test the hydrogen sorption properties of the ultrafine pure Mg and Mg-Ti powders [11]. The hydrogen desorption property of the hydrogenated powders was analyzed by thermogravimetric/differential thermal analysis spectroscope [(TG/DTA), Netzsch F449A Jupiter] under 0.1 MPa of argon at a heating rate of 10 K/min.
3 Results and discussion
3.1 Pure Mg ultrafine powders
Figure 2(a) shows a typical TEM image of the ultrafine pure Mg powders. It is revealed that most of the Mg particles are hexagonal in shape due to their crystallography symmetry. TEM observations in different areas show that the particle size of pure Mg is in the range of 50-700 nm. Similar types of particle morphology and size distribution were also observed in the ultrafine Mg-Ti powders. Figure 2(b) gives the XRD pattern of the ultrafine pure Mg powders after hydrogenation at 400 ℃. The hydrogenated powders consist of mainly MgH2, a small amount of MgO and residual Mg. The MgO is formed during passivation while the existence of the residual Mg indicates that some large Mg particles could not be fully hydrogenated [12]. Figure 3(a) shows the PCT curves of the ultrafine Mg powders measured at different temperatures. It is seen that the maximum hydrogen storage capacity and absorption/desorption plateaus of the powders increase by increasing the temperature. The maximum hydrogen storage capacity of ultrafine Mg particles at 400 ℃ is determined to be 6.24%. This value is slightly lower than 7.7% [13] of theoretical capacity of pure Mg due to the presence of MgO and residual Mg. The hydrogenation enthalpy of pure Mg powders was calculated to be -78.6 kJ/mol H2 from the PCT curves of hydrogen absorption plateau pressures according to the Van’t Hoff equation. This is very close to the value 74.5 kJ/mol H2 for pure Mg reported in Ref. [14]. Figure 3(b) shows the dynamic absorption profile of the ultrafine Mg powders measured at 400 ℃ under 4 MPa of hydrogen pressure. The powders can absorb 6% of hydrogen within 500 s, showing very good hydrogen absorption kinetic property. In contrast to the slow kinetics of the coarse grained Mg, this is attributed to the large surface area and short diffusion length of the ultrafine particles [15]. Figures 4(a) and (b) show the typical TEM images of a partially hydrogenated Mg particle and an un-hydrogenated large Mg particle in the Mg powders after hydrogen absorption at 400 ℃ and 4 MPa, respectively. It is seen in Fig. 4(a) that the partially hydrogenated Mg particle has a “core-shell” structure. That is, the outside shell, which is visible in a transparent manner, is MgH2. In contrast, the relative dark core is Mg that is still not hydrogenated yet. In Fig. 4(b), a large un-hydrogenated Mg particle is clearly visible, which is confirmed by the selected area electron diffraction (SAED) pattern shown as inset. These results show that some large Mg particles cannot be fully hydrogenated, in consistent with the results given above.
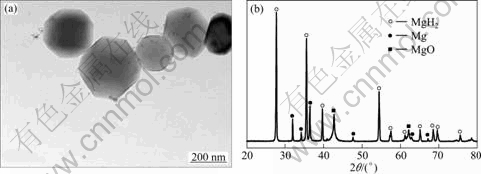
Fig. 2 Typical TEM image of ultrafine pure Mg powders (a) and XRD pattern of powders after hydrogenation at 400 ℃ (b)
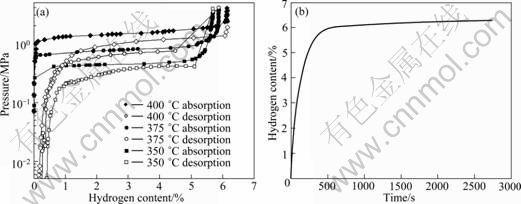
Fig. 3 PCT curves of pure Mg ultrafine powders measured at different temperatures (a) and dynamic absorption profile obtained at 400 ℃ under 4 MPa (b)

Fig. 4 Typical TEM images of partially hydrogenated Mg particle (a) and un-hydrogenated large Mg particle in Mg powders after hydrogen absorption at 400 ℃ and 4 MPa (b)
3.2 Mg-Ti ultrafine powders
Figure 5(a) gives the XRD pattern of the ultrafine Mg-Ti powders prepared by arc plasma method. The powders are composed of mainly Mg, a small amount of MgO and Ti. Considering that the measured Ti content in the powders is around 6.78%, the XRD analysis indicates that most of Ti is alloyed with Mg in solid solution state [16]. The Ti content in the ultrafine Mg-Ti powders is much lower than that in the initial mixture. Indeed, the evaporation temperature and latent heat of evaporation of Ti are much higher than those of Mg, leading to the much lower evaporation rate of Ti when compared with Ti. Figure 5(b) shows the PCT curves of the ultrafine Mg-Ti powders measured at different temperatures. Similar to what is observed in pure Mg, the maximum hydrogen storage capacity and absorption/desorption plateaus of the Mg-Ti powders also increase with increasing the temperature. The maximum hydrogen storage capacity of ultrafine Mg-Ti particles at 400 ℃ is found to be 4.3%, lower than the pure Mg ultrafine powders, which is attributed to the partial substitution of Mg by Ti and the existence of MgO and residual Mg particles. The hydrogenation enthalpy of the Mg-Ti powders was calculated to be -67 kJ/mol H2 from the PCT curves, which is much lower than that of the ultrafine pure Mg powders. This means that alloying with Ti can significantly improve the thermodynamic property of the ultrafine Mg powders. The effects of the addition of transition metals into Mg on the hydrogen sorption properties have been studied both experimentally and theoretically [14,17,18]. Based on the first principle calculations, the improved hydrogen sorption properties through partial substitution of Mg by 3d transition metals can be attributed to the destabilization of Mg-H bond due to interaction between valance electron of H and the unsaturated d electron shells of transition metals [19,20]. In the present case, the experimental results confirm that the partial substitution of Ti for Mg can reduce the hydrogenation enthalpy sorption in Mg.
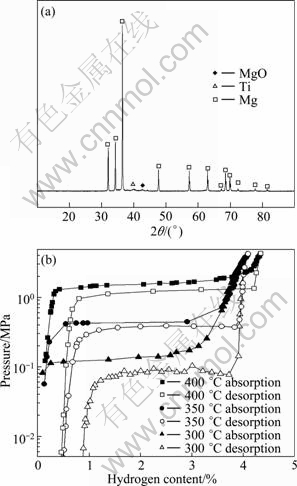
Fig. 5 XRD pattern of ultrafine Mg-Ti powders (a) and PCT curves of ultrafine Mg-Ti powders measured at different temperatures (b)
Figures 6(a) and (b) show the TG/DTA curves of the hydrided pure Mg and Mg-Ti powders obtained under 4 MPa hydrogen and 400 ℃, respectively. From Fig. 6(a), the onset dehydriding temperature for pure Mg is found to be 423 ℃ and the dehydriding is a one-step endothermic reaction from MgH2 to Mg+H2. In Fig. 6(b), it is observed from DTA curve that two endothermic peaks appear during the dehydriding of the ultrafine hydrogenated Mg-Ti powders. The corresponding TG curve confirmed the two-step dehydriding process of Mg-Ti-H powders. The onset dehydriding temperature for Mg-Ti-H is 386 ℃, much lower than that of MgH2 measured under same conditions. Comparing the DTA curves in Fig. 6(a) and (b), it is seen that the second peak of the Mg-Ti-H powders located at a quite similar temperature as that of the MgH2 powders. This phenomenon can be attributed to the local heterogeneous distribution of Ti in ultrafine Mg particles. Indeed, the Ti content in the Mg-Ti powders is quite low (6.78%). Some of the Mg particles may not contain Ti or local Ti content is fairly low. Those Mg particles should show similar hydriding and dehydriding behaviors as pure Mg, which are different from those Mg particles containing high content of Ti. Similar phenomena were also observed in other Mg-based alloys for hydrogen storage [17]. The above results prove that the addition of Ti into ultrafine Mg powders can also reduce the dehydriding temperature of Mg hydride.
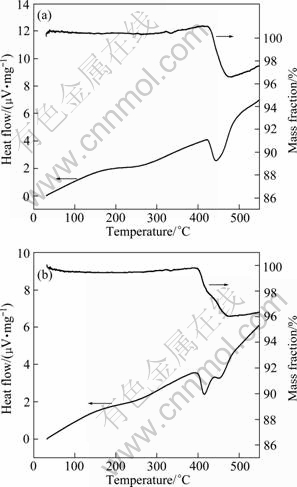
Fig. 6 TG/DTA curves of ultrafine hydrogenated pure Mg (a) and Mg-Ti (b) particles
4 Conclusions
1) An arc plasma method was used to produce ultrafine pure Mg and Mg-Ti particles for hydrogen storage. TEM observations reveal that most of the ultrafine Mg and Mg-Ti particles have a hexagonal shape. Their particle size is in the range of 50-700 nm.
2) Based on the hydrogen absorption plateau pressures measured by PCT, the hydrogenation enthalpy of Mg-Ti powders is determined to be about -67 kJ/mol H2, while it is -78.6 kJ/mol H2 measured for pure Mg powders.
3) After hydrogenation, some of the large Mg particles cannot be fully hydrogenated. This explains the fact that the maximum hydrogen storage capacity of the Mg powders is lower than the theoretical capacity of Mg.
4) The onset dehydriding temperature of hydrogenated Mg-Ti powders is measured to be 386 ℃; which is significantly lower than the dehydriding temperature 423 ℃ of hydrogenated pure Mg powders. Therefore, the addition of Ti into Mg through arc evaporation method can effectively improve the thermodynamic properties of Mg for hydrogen storage.
References
[1] DILLON A C, BEKKEDAHL T A, CAHILL A F, JONES K M, HEBEN M J. Carbon nanotube materials for hydrogen storage [C]//Proc US DOE Hydrogen Program Review, 1995: 521-541.
[2] ZUTTEL A. Materials for hydrogen storage [J]. Materials Today, 2003, 9: 24-33.
[3] SCHLAPBACH L, ZUTTEL A. Hydrogen-storage materials for mobile applications [J]. Nature, 2001, 414: 353-358.
[4] WAGEMANS R W P, van LENTHE J H, de JONGH P E, van DILLEN A J, de JONG K P. Hydrogen storage in magnesium clusters: Quantum chemical study [J]. Journal of the American Chemical Society, 2005, 127(47): 16675-16680.
[5] REISER A, BOGDANOVIC B, SCHLICHTE K. The application of mg-based metal-hydrides as heat energy storage systems [J]. International Journal of Hydrogen Energy, 2000, 25: 420-430.
[6] KOJIMA Y, KAWAI Y, HAGA T. Magnesium-based nano-composite materials for hydrogen storage [J]. Journal of Alloys and Compounds, 2006, 424: 294-298.
[7] ZALUSKA A, ZALUSKI L, STROM-OLSEN J O. Structure, catalysis and atomic reactions on the nano-scale: A systematic approach to metal hydrides for hydrogen storage [J]. Applied Physics A: Material Science and Processing, 2001, 72: 157-165.
[8] BARKHORDARIAN G, KLASSEN T, BORMANN R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg [J]. Journal of Alloys and Compounds, 2004, 364: 242-246.
[9] BAZZANELLA N, CHECCHETTO R, MIOTELLO A, SADA C, MAZZOLDI P, MENGUCCI P. Hydrogen kinetics in magnesium hydride; on different catalytic effects of niobium [J]. Applied Physics Letter, 2006, 89: 014101-1-3.
[10] SHAO H Y, WANG Y T, XU H R, LI X G. Hydrogen storage properties of magnesium ultrafine particles prepared by hydrogen plasma-metal reaction [J]. Materials Science and Engineering B, 2004, 110: 221-226.
[11] CHECCHETTO R, TRETTEL G, MIOTELLO R. Sievert-type apparatus for the study of hydrogen storage in solids [J]. Measurement Science and Technology, 2004, 15(1): 127-130.
[12] SCHIMMEL H G, KEARLEY G J, HUOT J, MULDER F M. Hydrogen diffusion in magnesium metal (α phase) studied by ab initio computer simulation [J]. Journal of Alloys and Compounds, 2005, 404-406: 235-237.
[13] ZALUSKA A, ZALUSKI L, STROM-OLSEN J O. Nanocrystalline magnesium for hydrogen storage [J]. Journal of Alloys and Compounds, 1999, 288: 217-225.
[14] JAIN I P. Hydrogen the fuel for 21st century [J]. International Journal of Hydrogen Energy, 2009, 34: 7368-7378.
[15] IMAMURA H, MASANARIK K, KUSUHARA M, KATSUMOTO H, SUMI T, SAKATA Y. High hydrogen storage capacity of nanosized magnesium synthesized by high energy ball-milling [J]. Journal of Alloys and Compounds, 2007, 386: 211-221.
[16] MURRAY J L. The MG-TI system [J]. Journal of Phase Equilibria, 1986, 7: 245-248.
[17] KELKAR T, PAL S. A computational study of electronic structure, thermodynamics and kinetics of hydrogen desorption from Al- and Si- doped α-, β-, and γ- MgH2 [J]. Journal of Materials Chemistry, 2009, 19: 4348-4355.
[18] HANADA N, ICHIKAWA T, FUJI H. Catalytic effect of nanoparticle 3d- transition metals on hydrogen storage properties in magnesium hydride mgh2 prepared by mechanical milling [J]. The Journal of Physical Chemistry B, 2005, 109(15): 7188-7194.
[19] POZZO M, ALFE D. Hydrogen dissociation and diffusion on transition metal (=Ti, Zr, V, Ru, Co, Pd, Cu, Ag) doped Mg (0001) surfaces [J]. International Journal of Hydrogen Energy, 2009, 34: 1922-1930.
[20] NOVAKOVIC N, NOVAKOVIC J G, MATOVIC L. Ab-initio calculations of MgH2, MgH2:Ti and MgH2:Co compounds [J]. Int J Hydrogen Energy, 2010, 35: 598-608.
超细纯镁及镁钛粉体的制备及储氢性能
李镇华1,邹建新1,2,曾小勤1,2,孙海全1,丁文江1,2
1. 上海交通大学 上海市镁材料及应用工程中心,轻合金精密成型国家工程中心,上海 200240;
2. 上海交通大学 材料科学与工程学院,金属基复合材料国家重点实验室,上海 200240
摘 要:采用直流电弧等离子体方法制备超细纯Mg及Mg-Ti粉体。运用 X射线衍射(XRD),透射电子显微镜(TEM),压力-成分-温度(PCT)方法和TG/DTA技术研究粉体吸放氢前后的相组成、微观结构和吸放氢性能。结果表明,大部分超细Mg和Mg-Ti颗粒呈六角形,颗粒大小在50-700 nm范围内。根据范特霍夫方程计算由PCT曲线获得的吸氢平台压力,Mg-Ti粉的氢化焓约为-67 kJ/mol H2,显著高于纯镁粉的氢化焓-78.6 kJ/mol H2。TG/DTA分析表明,氢化后Mg-Ti粉的放氢起始温度为386 ℃,低于氢化纯镁粉的放氢温度(423 ℃)。通过电弧蒸发法直接向Mg中添加Ti而获得的Mg-Ti超细粉体可以显著改善镁的储氢热力学性能。
关键词:Mg;Mg-Ti;直流电弧;超细颗粒;储氢
(Edited by LI Xiang-qun)
Foundation item: Project (10JC1407700) supported by the Key Basic Project from Science and Technology Committee of Shanghai, China; Project (11ZR1417600) supported by Shanghai Nature Science Foundation from Science and Technology Committee of Shanghai, China; Project(11PJ1406000) supported by ‘Pujiang’ project from the Science and Technology Committee of Shanghai, China; Project (12ZZ017) supported by Shanghai Education Commission, China; Project (20100073120007) supported by China Education Commission
Corresponding author: ZOU Jian-xin; Tel: +86-21-54742381; E-mail: zoujx@sjtu.edu.cn
DOI: 10.1016/S1003-6326(11)61396-4
Abstract: The ultrafine pure Mg and Mg-Ti particles were prepared through a direct current (DC) arc plasma method. The X-ray diffraction (XRD), transmission electron microscopy (TEM), pressure-composition-temperature (PCT) method and TG/DTA techniques were used to study the phase components, microstructure and hydrogen sorption properties of the powders before and after hydrogen absorption. It is revealed that most of the ultrafine Mg and Mg-Ti particles are hexagonal in shape with particle size in the range of 50-700 nm. According to the Van’t Hoff equation, the hydrogenation enthalpy of Mg-Ti powders is determined to be about -67 kJ/mol H2 based on the PCT curves of hydrogen absorption plateau pressures. This value is much higher than -78.6 kJ/mol H2 for pure Mg powders. TG/DTA analyses show that the onset dehydriding temperature of hydrogenated Mg-Ti powders is 386 ℃, which is significantly lower than that of the hydrogenated Mg (423 ℃). The results prove that the addition of Ti into Mg through arc evaporation method can improve the thermodynamic properties of Mg for hydrogen storage.


