J. Cent. South Univ. (2012) 19: 3378–3384
DOI: 10.1007/s11771-012-1417-3

Preparation and properties of composite polymer electrolyte modified with nano-size rare earth oxide
XIAO Wei(肖围), LI Xin-hai(李新海), GUO Hua-jun(郭华军), WANG Zhi-xing(王志兴), YANG Bo(杨波), WU Xian-wen(吴贤文)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
Poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) based composite polymer electrolyte (CPE) modified with CeO2, La2O3 and Y2O3 nano-rare earth oxides was prepared by phase inversion technique. Physical and chemical properties of the modified CPEs were studied by SEM, TG-DSC and electrochemical methods. The results show that the CPE modified with 10% La2O3 (mass fraction) has the best practical applicability, which indicates that the thermal and electrochemical stability can reach over 400°C and 4.5 V, respectively, and temperature dependence of ionic conductivity follows Vogel-Tamman-Fulcher (VTF) relationship and ionic conductivity at room temperature is up to 3.3 mS/cm. the interfacial resistance Ri reaches a stable value about 557 Ω after 6 d storage.
Key words:
1 Introduction
In order to overcome practical safety of traditional liquid electrolyte in lithium ionic battery, more and more attention has been paid to the novel polymer electrolyte due to its excellent advantages such as no-leakage of electrolyte, high energy density, flexible geometry and improved safety hazards [1–2]. Poly(vinylidene fluoride- co-hexafluoropropylene) (PVDF-HFP) is considered to be the most suitable matrix material for fabricating polymer electrolyte because PVDF-HFP has relatively low crystallinity due to the copolymerization effect between VDF and HFP and high ionic conductivity due to more amorphous region compared to other materials such as PVDF [3–5]. However, the mechanical performance of the PVDF-HFP based polymer electrolyte cannot meet the practical requirements. Adding the inorganic fillers such as silica (SiO2), copper oxide (CuO), titania (TiO2) and molecule sieve into the polymer matrix can improve the physical strength as well as increase the ionic conductivity due to the improved absorption level of electrolyte solution [6–10]. Except for these effects, they can act as a solid plasticizer hindering the reorganization of polymer chains and interact with polar groups by Lewis acid-base reaction [1, 2, 11]. So, the properties such as ionic conductivity,
lithium ions transference number and activation energy for ions transport can gain much improvement. Although inorganic fillers modified PVDF-HFP based composite polymer electrolyte (CPE) were reported in many literatures, there was less literature to report the CPEs modified by nano-rare earth oxides, which are widely used in the field of functional materials such as catalyst preparation, optical glass and magnetic materials due to their special physical and chemical properties [12–13]. In the present work, the novel PVDF-HFP based CPEs modified with nano-size CeO2, La2O3 and Y2O3 rare earth oxides are prepared by phase inversion technique. Physicochemical and electrochemical properties of nano- rare earth oxides doped PVDF-HFP based CPEs exhibit more distinguishing improvement compared with pure PVDF-HFP electrolyte. In order to investigate the mechanism about the CPEs in more detail, the distinguishing improvement is studied by SEM, TG-DSC, electrochemical impedance spectroscopy (EIS) and linear sweep voltammetry (LSV).
2 Experimental
2.1 Materials and preparation of CPEs
PVDF-HFP (Atofina, Kynar 2801) was used as polymer matrix in this work. Nano-size CeO2 (20–30 nm, 36–38 m2/g), La2O3 (20–30 nm, 25–28 m2/g) and Y2O3 (20–30 nm, 25–28 m2/g) rare earth oxides were purchased from Rare-Chem Hi-Tech Co, Ltd, China. PVDF-HFP and rare earth oxides were dried under vacuum for 12 h at 60 and 120°C prior to use, respectively. Both analytical grade solvent N,N-dimethylformamide (DMF) and pore-forming agent polyethylene glycol with low relative molecular mass of 200 g/mol (PEG-200) were directly used without further purification. At first, different contents of nano-size oxide were added to anhydrous ethanol with ultrasonic vibration for 3-4 h and a certain amount of PVDF-HFP was dissolved in DMF with continuous stirring, in which the mass ratios of nano-rare earth oxide to PVDF-HFP were 0.06, 0.08, 0.10, 0.12 and 0.14. Then, two kinds of the aforesaid solution were mixed with continuous vigorous stirring for 3-4 h at 40 °C. After forming homogeneous casting solution, the wet free-standing membrane was fabricated by casting with a doctor blade. And the desirable CPE membranes were obtained by conventional phase inversion technique. To prepare desirable CPEs, the as-prepared PVDF-HFP based CPE membranes must be activated by immersing into the 1.0 mol/L LiPF6-EC/DMC/EMC (1:1:1, volume ratio) liquid electrolyte solution at room temperature for 1 h in an argon-filled glove box to protect from moisture. Meanwhile, as a control sample, the PVDF-HFP based polymer electrolytes without nano-size rare earth oxide were also prepared under the same conditions in this work.
2.2 Properties characterizations
In order to measure the liquid electrolyte uptake ratio (A) of CPEs, the as-prepared electrolyte membranes with 14 mm in diameter were immersed into 1.0 mol/L LiPF6-EC/DMC/EMC (1:1:1, volume ratio) solution at room temperature for 1 h in an argon-filled glove box to activate. The liquid electrolyte uptake ratio (A) is calculated with Eq. (1), in which m1 and m0 are the masses of the wet and dry membranes, respectively:
 (1)
(1)
For morphological features of the CPEs membranes observation, a scanning electron microscope (SEM, JSM6301F) with an accelerating voltage of 20 kV was employed to examine the membrane surface sputter coated with gold under vacuum atmosphere. Thermogravimetry and differential scanning calorimeter (TG-DSC) measurements were carried out on a Perkin-Elmer Pyris-1 analyzer. The measurements were performed at a heating rate of 10°C /min from 40 to 400°C. A flow of nitrogen gas was maintained over the perforated pan to avoid any contact with atmospheric moisture. The sample mass was maintained in the range of 12-15 mg and an empty aluminum pan was used as a reference.
The ionic conductivity of the CPEs was determined by EIS according to Eq. (2), where σ is the ionic conductivity; R is the bulk resistance of the symmetrical cell; l and S are the thickness and the area of the specimen, respectively. The as-prepared electrolyte membranes were sandwiched between two stainless (SS) blocking electrodes to fabricate the SS/CPE/SS model cell. The EIS tests were measured over an AC excitation amplitude of 5 mV and frequency range from 1 Hz to 100 kHz at various temperatures (293–363 K, the cells were thermostated during measurements) using a CHI660b frequency response analyzer (Shanghai Chenhua, China).
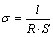 (2)
(2)
Electrochemical stability window of the CPEs was determined by running LSV in three-electrode cell using stainless steel as the blocking working electrode, lithium as both the counter and the reference electrode and polymer electrolyte as the electrolyte. The LSV tests were carried out using the same system as that in EIS at a scanning rate of 5 mv/s. Additionally, the interfacial stability was studied by investigating the interfacial resistance Ri change versus different storage time of the blocking model cell Li/ CPE/Li.
3 Results and discussion
3.1 Effect of nano-oxide content on electrolyte uptake ratio
The dependence of electrolyte uptake ratio of CPEs on the different contents of nano-rare earth oxides is shown in Fig. 1. It can be obviously seen from Fig. 1 that the electrolyte uptake ratios are affected by doping different kinds and contents of nano-rare earth oxide. Generally, the three kinds of nano-oxides demonstrate similar dependence on their doped contents. The electrolyte uptake ratio increases with increasing the doping contents of nano-rare earth oxide, which can reach the maximum at 10% (mass fraction) and then decreases with further increasing contents. Similar results are observed in PVDF-HFP based CPEs modified with other nano-inorganic fillers such as nano-Al2O3, and ZSM-5 [14–16]. It is obviously observed that the modified membrane with 10% La2O3 (mass fraction) shows the highest electrolyte uptake ratio up to 262% compared with CeO2 and Y2O3 modified membranes, which may be partly attributed to abundant micro-pores in the surface and inner of the modified membrane and partly to the Lewis acid-base interactions between the nano-rare earth oxide and the polymer matrix. The more elaboration presented in the following experiments is to study the practical performances of the PVDF-HFP based CPEs modified with 10 % nano-oxides.
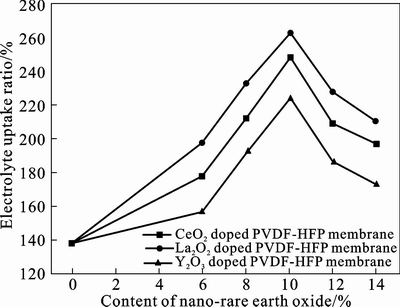
Fig. 1 Variation of electrolyte uptake ratio of CPEs with different contents of nano-oxides
3.2 SEM results
The SEM images of pure PVDF-HFP membrane and CPE membranes doped with nano-CeO2, nano-La2O3 and nano-Y2O3 are demonstrated in Fig. 2. Compared with pure PVDF-HFP membrane, the modified membranes display distinctly differences in term of morphology, which suggests that the added nano-rare earth oxides can play an important role to form the different surfaces. It is obviously observed that Fig. 2(c) presents the smoothest surface. Moreover, no aggregation is discovered on the surface of the CPE modified by ano-La2O3, which suggests that the added nano-La2O3 can be well dispersed. Well-dispersed nano-La2O3 and smooth surface can improve the interfacial properties between the electrolyte and electrodes, which can improve the cycle and rate performances of lithium ion battery. It is obviously observed that the micro-pore size of the nano-La2O3 modified membrane decreases with adding nano-oxide into the matrix and the abundant micro-pores in the inner-layer of the CPE demonstrate good internal connectivity, which can promote further more electrolyte entrapment rate proved by the above experiments. High electrolyte uptake ratio suggests high ionic conductivity, which indicates that the membrane modified with nano-La2O3 should exhibit high ionic conductivity.
3.3 Ionic conductivity
Ionic conductivity is considered to be the most important factor in the practical applications field of lithium ion battery, so the ionic conductivity of the as-prepared CPE membranes must be detailedly investigated. The reciprocal temperature dependence of ionic conductivity of the PVDF-HFP based polymer electrolyte with and without nano-rare earth oxides is presented in Fig. 3. According to Eq. (2), the ionic conductivity of the CPEs modified by 10% nano-CeO2, nano-La2O3 and nano-Y2O3 are 3.29, 3.58 and 2.94 mS/cm, respectively, whereas that of the pure PVDF- HFP polymer electrolyte is only 2.01 mS/cm at room temperature, which indicates that added nano-oxides can well improve the ionic conductivity of the CPEs. The increased ionic conductivity can be partly attributed to the more electrolyte uptake ratio of the nano-rare earth oxides modified CPEs (see Section 2.1) and partly to the interactions between the polymer matrix and added nano-oxides. Taking nano-CeO2 as an example, the modified CPE has the highest ionic conductivity mainly due to the interactions. On one hand, the Lewis acid character of the added nano-CeO2 would compete with the Lewis acid character of the lithium cations for the formation of complexes with the polymer matrix, which suggests that the polymer matrix would have more amorphous region for more Li+ transferring to improve the ionic conductivity; on the other hand, the nano-CeO2 would provide more physical cross-linking centers for the PVDF-HFP segments, which can lower the copolymer chain reorganization tendency and promote an overall structure stiffness. Such a structure modification would provide extra Li+ conducting pathways at the surface of nano-oxides, which accounts for the improvement in ionic transport.
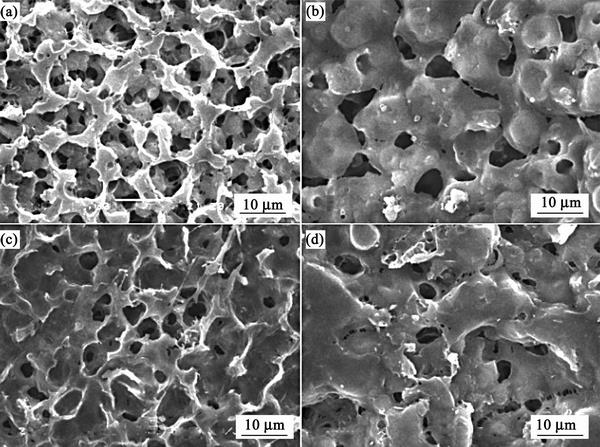
Fig. 2 SEM images of pure PVDF-HFP membrane (a) and CPE membranes doped with nano-CeO2 (b), nano-La2O3 (c) and nano-Y2O3 (d)
In order to further understand the ionic conduction mechanism of the CPEs, the ionic conductivity of the CPEs with 10% different nano-rare earth oxides is investigated in different temperature ranges from 293 to 363 K in Fig. 3. It is obviously seen that the ionic conductivity demonstrates the same dependence in all the temperature ranges, in which the ionic conductivity increases with increasing the temperature confirmed by other inorganic fillers [7, 11, 15, 17]. However, the ionic conductivity is not related linearly to the reciprocal temperature, which is different from the reported CPEs modified with some inorganic fillers and may obey the Vogel-Tamman-Fulcher (VTF) relation [18–19]. As the temperature increases, the polymer can expand easily and produce free volume. Thus, as temperature increases,the free volume increases. The resulting conductivity, representing the overall mobility of ions and the polymer, is determined by the free volume around the polymer chains. Therefore, as temperature increases, ions, solvated molecules, or polymer segments can move into the free volume. This leads to an increase in ion mobility and segmental mobility that will assist ion transport and virtually compensate for the retarding effect of the ion clouds.
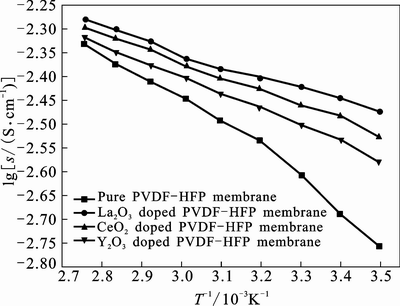
Fig. 3 Temperature dependence of ionic conductivity of PVDF- HFP based polymer electrolyte with and without nano-rare earth oxides
3.4 Thermal and electrochemical stability
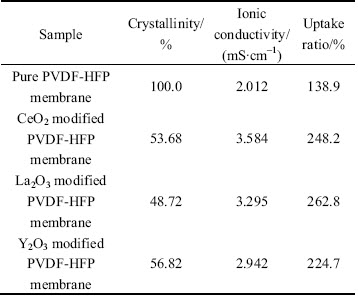
The TG-DSC plots of different PVDF-HFP based polymer electrolyte membranes prepared with and without nano-rare earth oxides by phase inversion method are shown in Fig. 4. For convenience, we define the decomposition temperature of the polymer electrolyte membrane at the mass loss larger than 1% of its original mass in our work. It is obviously observed in Fig. 4(a) that the polymer electrolyte membrane has higher thermal stability with adding nano-rare earth oxides into matrix. The tendency of TG curves of the nano-oxide modified membranes is similar to that of the pure PVDF-HFP electrolyte membrane, but the decomposition temperature is significantly improved from 335°C of the pure PVDF-HFP electrolyte membrane, to 360°C of nano-CeO2 and nano-Y2O3 modified membranes, to over 400°C of nano-La2O3 modified membrane, which suggests that the added nano-oxides can improve the thermal stability of the CPEs. Figure 4(b) shows the DSC plots of the four different kinds of membranes. According to the following relation x=△Hf/ΔHmfΘ from the DSC curves, where △Hf is the crystalline melting heat of PVDF (104.7 J/g), and △HmfΘ is the heat of the fusion of PVDF-HFP based polymer electrolyte membrane [20–21], the crystallinity of the membranes calculated according to the above equation is listed in the Table 1, together with the electrolyte uptake ratio and ionic conductivity at room temperature.
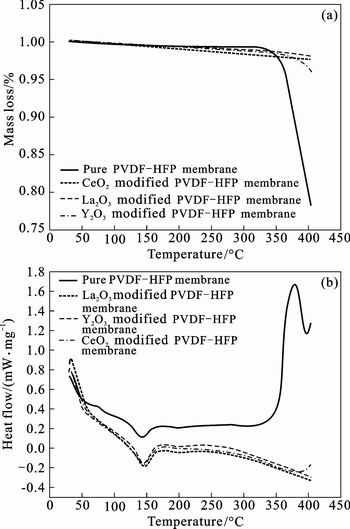
Fig. 4 Plots of TG (a) and DSC (b) about different kinds PVDF-HFP based polymer electrolyte with and without nano-rare earth oxides
As demonstrated in Table 1, it is reasonable to believe that the added nano-rare earth oxides play a positive role in improving ionic conductivity. The increased ionic conductivity of the nano-oxides modified membranes can be attributed to the decreasing crystallinity and increasing electrolyte uptake ratio, which is consistent with the results of Section 2.1 and 2.3.
Table 1 Results of crystallinity, ionic conductivity and electrolyte uptake ratio about different polymer electrolyte membranes
The plots of electrochemical stability window about different kinds the PVDF-HFP based polymer electrolytes` with and without nano-rare earth oxides at room temperature are demonstrated in Fig. 5. The nano-La2O3 modified PVDF-HFP membrane displays the highest electrochemical stability window about 4.5 V, while the electrochemical stability window of the pure PVDF-HFP membrane is about 4.0 V, which implies that
the nano-La2O3 can improve the electrochemical stability. This may be due to the interactions between the polymer matrix and nano-rare earth oxides, which can result in complex forming of polymer-rare earth.
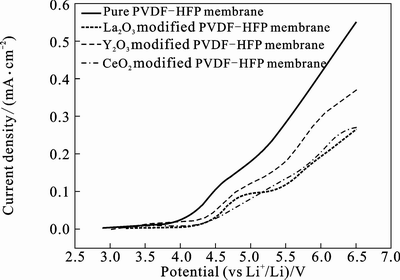
Fig. 5 Plots of LSV about different kinds PVDF-HFP based polymer electrolytes with and without nano-rare earth oxides
3.5 Interfacial properties
Interfacial compatibility between polymer electrolyte and electrodes is another important factor to ensure acceptable safety and cyclic performances in practical applications. In the polymer electrolyte system, the passive layer between the electrodes and electrolyte grows with the time, but the uncontrolled layer plays a vital role in practical applications [4, 11, 22]. In order to understand the interfacial stability between electrodes and electrolyte membranes, EIS of the fabricated symmetric Li/CPE/Li cell at open circuit was monitored with various storage times.
Nyquist plots of four different kinds of Li/As-prepared electrolyte/Li cells with various storage time are shown in Fig. 6. It is obviously observed that the interfacial resistances Ri of as-prepared membranes increase with increasing storage time due to the constantly increasing interfacial reactions. However, the increase tendency of Ri value of membrane with nano-rare earth oxides is much slower, which implies that nano-oxide can be used to improve the interfacial properties. The Nyquist diameter of the pure PVDF-HFP membrane keeps increasing after 6 d storage in Fig. 6(a), while the one of the modified membranes reaches a steady value under the same storage condition. For example, the Ri value of the pure PVDF-HFP membrane increases from 1 020 Ω on the first day to 4 670 Ω after 6 d storage, whereas the nano-La2O3 modified PVDF- HFP membrane increases only from 382 Ω on the first day to a steady value about 557 Ω after 6 d storage. The results indicate that, on one hand, the passive film forms between the electrolyte and electrodes at the beginning and reaches a stable value when adding the nano-oxides to the polymer matrix, thus the membranes modified in nano-oxides exhibit good compatibility with the electrode [22], which can be strongly proved in Section 2.2; on the other hand, the added nano-rare earth oxides with high surface area can trap any impurities such as water and trace organic solvent due to the capillarity, inhibiting the destructive reaction on the electrode, which can improve the compatibility between the electrodes and electrolyte membrane [10, 22]. The further reaction mechanism about nano-rare earth oxides and polymer matrix on the modified membranes’surface still needs further investigation.
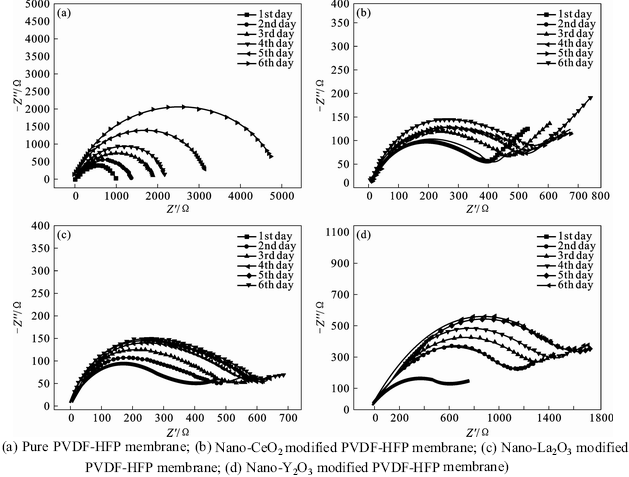
Fig. 6 Nyquist plots of different Li/As-prepared electrolyte/Li cells with various storage times:
4 Conclusions
1) CPEs modified with different contents of CeO2, La2O3 and Y2O3 nano-rare earth oxides are prepared by phase inversion technique and the CPEs with 10% nano-rare earth oxides have the highest electrolyte uptake rate.
2) The CPE modified with 10% La2O3 shows best practical applicability, in which thermal stability can achieve over 400°C, electrochemical stability window increases to 4.5 V and ionic conductivity at ambient temperature is up to 3.3 mS/cm-1.
3) Reciprocal temperature dependence of ionic conductivity of the CPE modified with 10% La2O3 follows VTF relationship and the interfacial resistance Ri can reach a steady value about 557 Ω after only 6 d storage, which can be attributed to the improved interfacial properties with 10% La2O3 added into the polymer matrix.
References
[1] CROCE F, SACCHETTI S, SCROSATI B. Advanced lithium batteries based on high-performance composite polymer electrolytes [J]. Journal of Power Sources 2006, 162(1): 685-689.
[2] SONG J Y, WANG Y Y, WAN C C. Review of gel-type polymer electrolytes for lithium-ion batteries [J]. Journal of Power Sources, 1999, 77(2): 183-197.
[3] ferrari s, quartarone e, mustarelli p, magistris a, fagnoni m, protti s, gerbaldi c, spinella a. lithium ion conducting pvdf-hfp composite gel electrolytes based on n- methoxyethyl-n-methylpyrrolidinium bis (trifluoromethanesulfonyl) -imide ionic liquid [j]. journal of Power Sources, 2010, 195(2): 559-566.
[4] LIU F, HASHIM N A, LIU Y, ABED M R M, LI K. Progress in the production and modification of PVDF membranes [J]. Journal of Membrane Science, 2011, 375(1, 2): 1-27.
[5] MIAO R, LIU B, ZHU Z, LIU Y, Li J, WANG X, LI Q. PVDF-HFP-based porous polymer electrolyte membranes for lithium-ion batteries [J]. Journal of Power Sources, 2008, 184(2): 420-426.
[6] CAILLION-CARAVANIER M, CLAUDE-MONTIGNY B, LEMORDANT D, BOSSER G. Absorption ability and kinetics of a liquid electrolyte in PVDF-HFP copolymer containing or not SiO2 [J]. Journal of Power Sources, 2002, 107(1): 125-132.
[7] KIM K M, PARK N G, RYU K S, CHANG S H. Characteristics of PVdF-HFP/TiO2 composite membrane electrolytes prepared by phase inversion and conventional casting methods [J]. Electrochimica Acta, 2006, 51(26): 5636-5644.
[8] KOH M J, HWANG H Y, KIM D J, KIM H , HONG Y T, NAM S Y.
Preparation and characterization of porous PVDF-HFP/clay nanocomposite membranes [J]. Journal of Materials Science & Technology, 2010, 26(7): 633-638.
[9] PRINGLE J M, SHEKIBI Y, MACFARLANE D R, FORSYTH M. The influence of different nanoparticles on a range of organic ionic plastic crystals [J]. Electrochimica Acta, 2010, 55(28): 8847-8854.
[10] REDDY M J, CHU P P. 7Li NMR spectroscopy and ion conduction mechanism in mesoporous silica (SBA-15) composite poly(ethylene oxide) electrolyte [J]. Journal of Power Sources, 2004, 135(1/2): 1-8.
[11] CROCE F, PERSI L, RONCI F, SCROSATI B. Nanocomposite polymer electrolytes and their impact on the lithium battery technology [J]. Solid State Ionics, 2000, 135(1/2/3/4): 47-52.
[12] KHADIJEH RES, ELIAS S B, WOOD A K, REZA A M. Rare earth elements distribution in marine sediments of Malaysia coasts [J]. Journal of Rare Earths, 2009, 27(6): 1066-1071.
[13] XIAO W, MAN R, MIAO C, PENG T. Study on corrosion resistance of the BTESPT silane cooperating with rare earth cerium on the surface of aluminum-tube [J]. Journal of Rare Earths, 2010, 28(1): 117-122.
[14] SARNOWSKA A, POLSKA I, NIEDZICKI L, MARCINEK M, ZALEWSKA A. Properties of poly(vinylidene fluoride-co- hexafluoropropylene) gel electrolytes containing modified inorganic Al2O3 and TiO2 filler, complexed with different lithium salts [J]. Electrochimica Acta, 2001, 57(8): 180–186
[15] XI J, QIU X, WANG J, BAI Y, ZHU W, CHEN L. Effect of molecular sieves ZSM-5 on the crystallization behavior of PEO-based composite polymer electrolyte [J]. Journal of Power Sources. 2006; 158(1): 627–634.
[16] XI J, TANG X. Investigations on the enhancement mechanism of inorganic filler on ionic conductivity of PEO-based composite polymer electrolyte: The case of molecular sieves [J]. Electrochimica Acta, 2006, 51(22): 4765-4770.
[17] JIANG Y X, CHEN Z F, ZHUANG Q C, XU J M, DONG Q F, HUANG L, SUN S G. A novel composite microporous polymer electrolyte prepared with molecule sieves for Li-ion batteries [J]. Journal of Power Sources, 2006, 160(2): 1320-1328.
[18] TOMINAGA Y, SHIMOMURA T, NAKAMURA M. Alternating copolymers of carbon dioxide with glycidyl ethers for novel ion-conductive polymer electrolytes [J]. Polymer, 2010, 51(19): 4295-4298.
[19] YOSHIDA K, MANABE H, TAKAHASHI Y, FURUKAWA T. Correlation between ionic and molecular dynamics in the liquid state of polyethylene oxide/lithium perchlorate complexes [J]. Electrochimica Acta, 2011, 57(8): 139–146.
[20] APPETECCHI G B, CROCE F, SCROSATI B. Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes [J]. Electrochimica Acta, 1995, 40(8): 991-997.
[21] JIA P, YEE WA, XU J, TOH CL, MA J, LU X. Thermal stability of ionic liquid-loaded electrospun poly(vinylidene fluoride) membranes and its influences on performance of electrochromic devices [J]. Journal of Membrane Science, 2011, 376(1/2): 283-289.
[22] TARASCON J M, GOZDZ A S, SCHMUTZ C, SHOKOOHI F, WARREN P C. Performance of Bellcore’s plastic rechargeable Li-ion batteries [J]. Solid State Ionics, 1996, 86–88(Part 1): 49-54.
(Edited by HE Yun-bin)
Foundation item: Project(2011FJ1005) supported by the Major Provincial Science and Technology Program of Hunan Province, China; Project (2010qzzd0101) supported by the Central College on the 2010 Operational Costs of Basic Research Program, China
Received date: 2011-12-28; Accepted date: 2012-06-24
Corresponding author: LI Xin-hai, Professor, PhD; Tel: +86–731–88836633; E-mail: xwylyq2006@126.com
Abstract: Poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) based composite polymer electrolyte (CPE) modified with CeO2, La2O3 and Y2O3 nano-rare earth oxides was prepared by phase inversion technique. Physical and chemical properties of the modified CPEs were studied by SEM, TG-DSC and electrochemical methods. The results show that the CPE modified with 10% La2O3 (mass fraction) has the best practical applicability, which indicates that the thermal and electrochemical stability can reach over 400°C and 4.5 V, respectively, and temperature dependence of ionic conductivity follows Vogel-Tamman-Fulcher (VTF) relationship and ionic conductivity at room temperature is up to 3.3 mS/cm. the interfacial resistance Ri reaches a stable value about 557 Ω after 6 d storage.


