Trans. Nonferrous Met. Soc. China 24(2014) 3696-3701
Calculating models on surface tension of RE2O3-MgO-SiO2 (RE=La, Nd, Sm, Gd and Y) melts
Cheng-chuan WU, Guo-guang CHENG
State Key Laboratory of Advanced Metallurgy, University of Science and Technology Beijing, Beijing 100083, China
Received 23 November 2013; accepted 26 June 2014
Abstract:
A thermodynamic model was developed for determining the surface tension of RE2O3-MgO-SiO2 (RE=La, Nd, Sm, Gd and Y) melts considering the ionic radii of the components and Butler’s equation. The temperature and composition dependence of the surface tensions in molten RE2O3-MgO-SiO2 slag systems was reproduced by the present model using surface tensions and molar volumes of pure oxides, as well as the anionic and cationic radii of the melt components. The iso-surface tension lines of La2O3-MgO-SiO2 slag melt at 1873 K were calculated and the effects of slag composition on the surface tension were also investigated. The surface tensions of La2O3, Gd2O3, Nd2O3 and Y2O3 at 1873 K were evaluated as 686, 677, 664 and 541 mN/m, respectively. The surface tension of pure rare earth oxide melts linearly decreases with increasing cationic field strength, except for Y2O3 oxide, while Y2O3 has a much weaker surface tension. The evaluated results of the surface tension show good agreements with literature data, and the mean deviation of the present model is found to be 1.05% at 1873 K.
Key words:
rare-earth oxide; surface tension; ionic radius; Butler’s equation; calculating model;
1 Introduction
Physical properties of elevated temperature melts are important parameters for high temperature related industries, such as iron and steel making, glass melting, ceramics sintering, controlling the rate of various reactions and the fluid flows [1]. Moreover, over the last few decades, since rare-earth elements including Sc, Y and lanthanides (Ln) have a common ionic valence (Z) of +3, and are known to be the most electropositive elements, it has been suggested that they are suitable sintering additives for Si3N4 [2]. Because the ionic radii (r) of the lanthanide elements decrease continuously with their increasing atomic number, which is known as lanthanide contraction, their cationic field strength (σCFS=Z/r2, Z is the valence of the corresponding element, r is its ionic radius based on r(VI O2-)=0.14 nm) also continuously changes with their atomic number. This causes consequent variations in various properties of rare-earth containing oxide and oxynitride glasses [3-9].
SHIMIZU et al [3] reported that the surface tension of rare earth (Y, Gd, Nd and La) containing 45.2%MgO-54.8%SiO2 (mole fraction) melts linearly decreases with the cationic field strength of RE3+. Conversely, the viscosity of rare earth (Y, Gd, Nd and La) containing 45.2%MgO-54.8%SiO2 (mole fraction) melts linearly increases with the cationic field strength of RE3+. The similarly linear relationship between rheological and elastic properties and cationic field strength of corresponding rare earth elements was also observed in RE-Si-Mg-O-N glasses (RE=Sc, Y, La, Nd, Sm, Gd, Yb and Lu) [4,5] and the effect of rare earth on the thermal expansion and viscosity properties of soda- lime-silica was investigated [7-9]. However, it is difficult to find the appropriate surface tension data of elevated temperature melts due to the high temperature measurement and the complicated effect of components on surface tension.
Various models have been developed to predict the surface tension of molten slag melts. For slag with complicated interactions, TANAKA et al [10-12] applied a model considering the anionic and cationic radii of the component oxides as the model parameters to describe the surface tension, CHOI and LEE [13] calculated the surface tension using critically evaluated ionic surface distances of pure oxides. In addition, these two models were based on Butler’s equation and achieved consistent results compared to the data reported in the literature. However, to our best knowledge, there are no available reports on the surface tension evaluation model of rare-earth containing high temperature silicate melts.
Therefore, in order to systematically reveal the influence of rare earth on the surface tension of silicate melts, a thermodynamic model for determining the surface tension of RE2O3-MgO-SiO2 (RE=La, Nd, Sm, Gd and Y) melts considering the ionic radii of the components and Butler’s equation was developed. The temperature and composition dependence of the surface tensions in molten RE2O3-MgO-SiO2 melts was reproduced by the present model using surface tensions and molar volumes of pure oxides, as well as the anionic and cationic radii of the melt components. The calculated surface tensions were compared with the experimentally obtained data for RE2O3-MgO-SiO2 melts. In addition, the surface tensions of pure oxides of RE2O3 at 1873 K were evaluated and the iso-surface tension lines of La2O3-MgO-SiO2 melts at 1873 K were also calculated.
2 Thermodynamic model for estimation of surface tension of molten slag
2.1 Butler’s equation
Among various models suggested by previous researchers for the prediction of surface tension of liquid solutions, the present model is also based on the Butler’s equation [14]. The surface tension (σ) of the molten slag system is calculated from
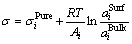 (1)
(1)
where subscript “i” refers to RE2O3, MgO or SiO2; superscripts “Surf” and “Bulk” indicate the surface and bulk phases, respectively; R is the gas constant and T is the temperature, 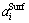 and
and 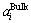 are the activities of the component i at the surface phase and the bulk phase, respectively;
are the activities of the component i at the surface phase and the bulk phase, respectively; 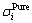 is the surface tension of the pure molten component i; and Ai is the molar surface area in a monolayer of pure molten component i, which can be figured out by
is the surface tension of the pure molten component i; and Ai is the molar surface area in a monolayer of pure molten component i, which can be figured out by
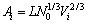 (2)
(2)
where N0 is Avogadro’s number, Vi is molar volume of the pure molten component i, L is correction factor resulted from the surface structure and is usually set to be 1 for the molten salts and ionic oxide mixtures by TANAKA et al [1].
2.2 Hypotheses
Equations (3)-(7) for evaluating the surface tension of the AX-BY-CZ melts have been derived from Butler’s equation by considering the following assumptions [10-12]:
1) It has been known that molten ionic mixtures readily undergo surface relaxation due to spontaneous changes in the ionic distance at the surface, which causes the energetic state of the surface to approach that of the bulk state. Thus, the contribution from excess Gibbs energy terms is neglected in the Butler’s equation.
2) In ionic substances, it is well known that their ionic structures depend upon the radii ratio of the cation to anion. In order to evaluate the ionic structures and physico-chemical properties of ionic materials, radii ratio of the cation to anion should be considered.
The relation between surface tension of slag melts and the Mi (substitute for the activities ai) of components at the surface phase and the bulk phase conforms to Butler’s equation:
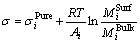 (3)
(3)
where 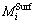 and
and 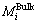 are the substitutes for the activities of the component i at the surface phase and the bulk phase, respectively. Taking CaO-Al2O3-CaF2 slag melt as an example, Eq. (3) can be expressed as Eqs. (4)-(6):
are the substitutes for the activities of the component i at the surface phase and the bulk phase, respectively. Taking CaO-Al2O3-CaF2 slag melt as an example, Eq. (3) can be expressed as Eqs. (4)-(6):
 (4)
(4)
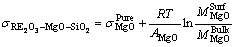 (5)
(5)
 (6)
(6)
where

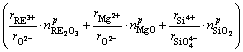 (7)
(7)
Subscript i refers to RE2O3, MgO or SiO2, subscripts “A” and “X” refer to the cations and anions of component i, respectively,  is the mole fraction of the component i in phase p (p=Surf or Bulk), rA is the radius of the cation, and rX is the radius of the anion. For example:
is the mole fraction of the component i in phase p (p=Surf or Bulk), rA is the radius of the cation, and rX is the radius of the anion. For example:
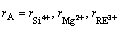 (8)
(8)
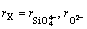 (9)
(9)
where 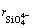 is considered to be the minimum anionic unit in SiO2, and the value of
is considered to be the minimum anionic unit in SiO2, and the value of 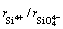 was experimentally determined to be 0.5 [1].
was experimentally determined to be 0.5 [1].
Data on the ionic radii were obtained from SHANNON et al [15,16], and the molar volumes of the pure oxides recommended by MILLS and KEENE [17] were used in the present model. These values are listed in Tables 1 and 2, respectively. The temperature dependences of the surface tension for pure RE2O3, MgO and SiO2 were collected from Refs. [10,18,19], as listed in Table 3.
Table 1 Radii of cationic and anionic ions
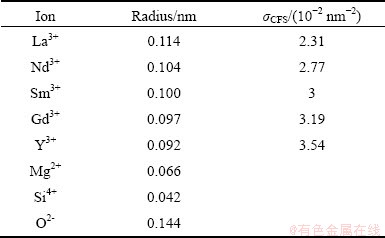
Table 2 Temperature dependence of molar volume of pure components

Table 3 Temperature dependence of surface tension of pure components

2.3 Model establishing
Based on the above study, the sequence of the established thermodynamic model for predicting the surface tension is shown in Fig. 1. As shown in Fig. 1, the parameter T is the absolute temperature, and ni are the mole fractions of three components as RE2O3, MgO and SiO2 of RE2O3-MgO-SiO2 melts to represent chemical composition of the slags. In addition, 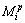 represents the relation of mole fraction of components, that is Eq. (7). Parameters
represents the relation of mole fraction of components, that is Eq. (7). Parameters 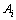 and
and 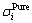 used in the calculation are listed in Tables 2 and 3.
used in the calculation are listed in Tables 2 and 3.
Figure 1 shows the sequences that 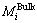 can be calculated by mole fractions (or mass fraction) of components and Eq. (7). Further, σ and
can be calculated by mole fractions (or mass fraction) of components and Eq. (7). Further, σ and 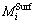 can be calculated by
can be calculated by 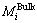 ,
, 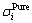 and
and 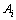 based on Eq. (7) and the Butler’s equation.
based on Eq. (7) and the Butler’s equation.
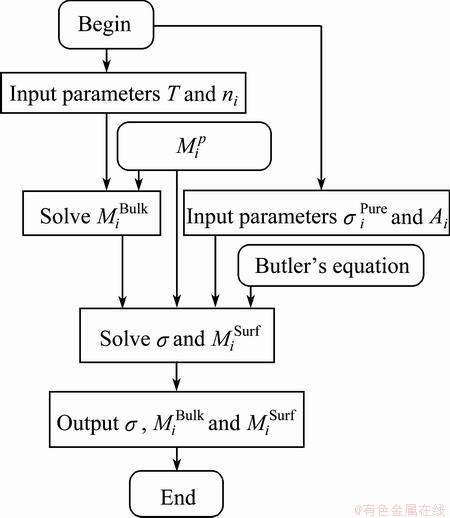
Fig. 1 Sequence of model for estimation of surface tension
The model stated above can be extended to multi-component slag systems, so long as the bulk phase and the surface phase both obey Eq. (7). Sample calculations have been made for ternary RE2O3-MgO-SiO2 slag melts.
3 Results and discussion
At a certain temperature, the calculation could be carried out with certain slag components. After linearization, Newton iterative method was used in Matlab software to gain all the surface tension of RE2O3-MgO-SiO2 slag melts.
3.1 Evaluating surface tension of RE2O3 at 1873 K
Since this calculating model has been verified in a lot of ternary molten slags, which includes not only such oxides as SiO2, Al2O3, CaO, FeO, MgO and MnO, but also surface-active components such as Na2O, B2O3 and CaF2, the surface tensions of RE2O3 (RE=Y, Gd, Nd and La) at 1873 K are derived based on this calculating model and the surface tension of three different compositions of RE2O3-MgO-SiO2 slag melts [3] at 1873 K. The surface tensions of Y2O3, Nd2O3, Gd2O3 and La2O3 are 541, 664, 677 and 686 mN/m, respectively, as shown in Table 3 and Fig. 2. In Fig. 2, the surface tensions of pure RE2O3 melts at 1873 K are plotted as a function of the cationic field strength of RE3+. Figure 2 obviously reveals that the surface tension of pure rare earth oxide melts linearly decreases with increasing cationic field strength, except Y2O3 oxides, while Y2O3 has a much weaker surface tension. The surface tensions obviously increase in the order of cationic radius of rare-earth (Table 3) from Y2O3, Gd2O3, and Nd2O3 to La2O3. Figure 3 shows the temperature dependences of the surface tension of pure RE2O3 melts.
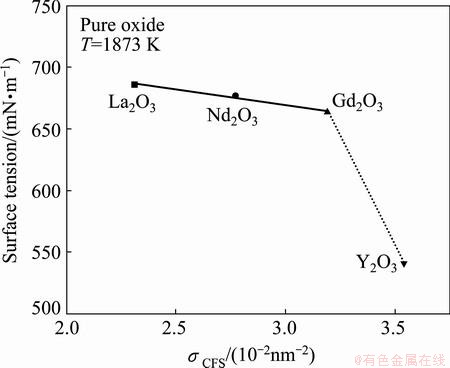
Fig. 2 Surface tension of pure RE2O3 melts at 1873 K
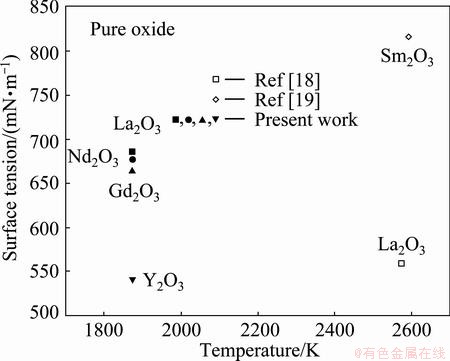
Fig. 3 Temperature dependences of surface tension of pure RE2O3 melts
LOFAJ et al [4,5] investigated the structure and physical properties (hardness, glass transition temperature and density of Me-Mg-Si-O-N (Me=Sc, Lu, Yb, Y, Sm and La) oxynitride glasses and reported that these properties varied linearly with radius of the Me3+ cation, moreover, depending on whether Me3+ belongs to the 3rd group (Sc and Y) of the periodic table of elements or the lanthanides, there were two distinct correlations. They have estimated that the deviations of physical properties from linearity were basically attributed to the considerable difference of atomic mass between lanthanide and non-lanthanide elements. However, in the present work, it is questionable whether the atomic mass will affect the surface tension of elevated temperature melts. This is experimentally evident that not only the cation size by itself but also the electronic structure of RE3+ cation has an influence on physical properties of high temperature melt. Furthermore, it is probable that the rare-earth oxide melts have a quite different microstructure in bulk and at surface.
3.2 Model evaluation
In order to evaluate the performance of the present model, the mean deviation is defined as
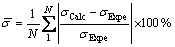 (10)
(10)
where 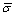 is the mean deviation, σCalc and σExpe [3] are the calculated and measured surface tensions, respectively, and N represents the number of the samples.
is the mean deviation, σCalc and σExpe [3] are the calculated and measured surface tensions, respectively, and N represents the number of the samples.
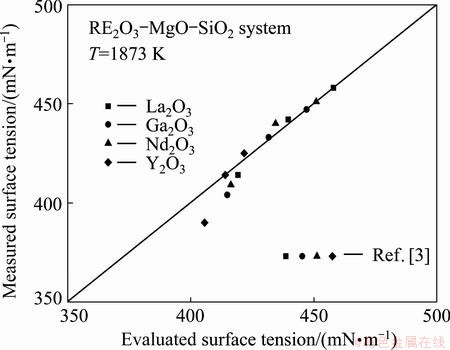
Fig. 4 Comparison of evaluated and measured surface tension for RE2O3-MgO-SiO2 system
Table 4 Comparison of evaluated and measured surface tensions for RE2O3-MgO-SiO2 system
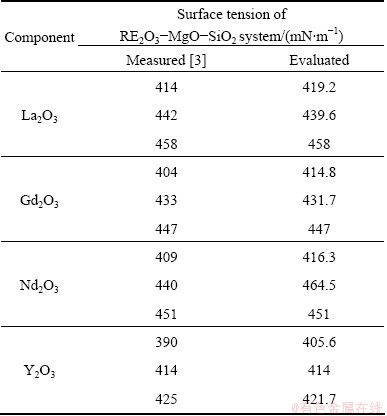
The surface tension data of melts of three different compositions in the system RE2O3-MgO-SiO2 (RE=La, Nd, Gd and Y) were measured in the early publication [3], which has been well represented by the present model. The comparison of the results between estimated surface tension data and the experimental surface tension data is shown in Fig. 4 and Table 4. The mean deviation computed by Eq. (10) is found to be 1.05% at 1873 K. Therefore, the present model provides a basical description of the surface tension variation of the RE2O3-MgO-SiO2 system with regard to temperature and composition.
3.3 Model application
The calculated results for the iso-surface tension of the La2O3-MgO-SiO2 system at 1873 K are shown in Fig. 5. These results are in good agreements with the literature values [3]. The iso-surface tension curves in Fig. 5, calculated using the current model, reproduce the composition dependence of surface tension for the La2O3-MgO-SiO2 system, and show that its surface tension increases with the increase of La2O3 content and MgO content and decreases as SiO2 content increases.
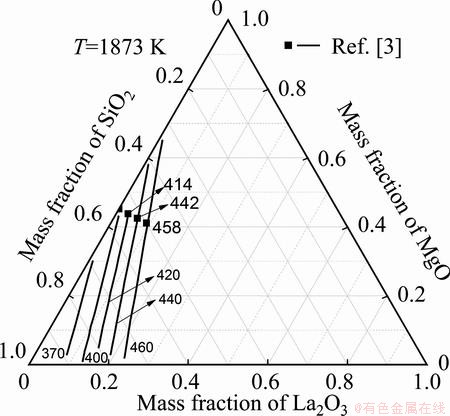
Fig. 5 Calculated iso-surface tension lines of La2O3-MgO-SiO2system at 1873 K
4 Conclusions
1) Based on the ionic radii of the components and Butler’s equation, a calculating model is developed for determining the surface tension of RE2O3-MgO-SiO2 molten slags.
2) The surface tensions of La2O3, Gd2O3, Nd2O3 and Y2O3 at 1873 K are evaluated as 686, 677, 664 and 541 mN/m, respectively. The surface tension of pure rare earth oxide melts linearly decreases with increasing cationic field strength, except Y2O3 oxides, whish has a much weaker surface tension.
3) The evaluated results for the surface tension from the present model show good agreements with literature values in RE2O3-MgO-SiO2 ternary system. The mean deviation of the present model is found to be 1.05% at 1873 K.
4) The iso-surface tension lines of La2O3-MgO- SiO2 slag melt are calculated. Surface tension of La2O3-MgO-SiO2 slag decreases with increasing SiO2 content and increases with increasing La2O3 content and MgO content.
References
[1] TANAKA T, KITAMURA T, BACK I A. Evaluation of surface tension of molten ionic mixtures [J]. ISIJ International, 2006, 46(3): 400-406.
[2] NEGITA K. Ionic radii and electro negativities of effective sintering aids for Si3N4 ceramics [J]. Journal of Materials Science Letters, 1985, 4(4): 417-418.
[3] SHIMIZU F, TOKUNAGA H, SAITO N, NAKASHIMA K. Viscosity and surface tension measurements of RE2O3-MgO-SiO2 (RE=Y, Gd, Nd and La) melts [J]. ISIJ International, 2006, 46(3): 388-393.
[4] LOFAJ F, SATET R, HOFFMANN M J, de ARELLANO LOOEZEZ A R. Thermal expansion and glass transition temperature of the rare-earth doped oxynitride glasses [J]. Journal of the European Ceramic Society, 2004, 24(12): 3377-3385.
[5] LOFAJ F, DERIANO S, LEFLOCH M, ROUXEL T, HOFFMANN M J. Structure and rheological properties of the RE-Si-Mg-O-N (RE=Sc, Y, La, Nd, Sm, Gd, Yb and Lu) glasses [J]. Journal of Non-Crystalline Solids, 2004, 344(1-2): 8-16.
[6] EL-OKR M, IBRAHEM M, FAROUK M. Structure and properties of rare-earth-doped glassy systems [J]. Journal of Physics and Chemistry of Solids, 2008, 69(10): 2564-2567.
[7] WANG Mi-tang, CHENG Jin-shu, LI Mei. Effect of rare earths on viscosity and thermal expansion of soda-lime-silicate glass [J]. Journal of Rare Earths, 2010, 28(S1): 308-311.
[8] WANG Mi-tang, CHENG Jin-shu. Viscosity and thermal expansion of rare earth containing soda-lime-silicate glass [J]. Journal of Alloys and Compounds, 2010, 504(1): 273-276.
[9] HAMPSHIRE S, POMEROY M J. Effect of composition on viscosity of rare earth oxynitride glasses [J]. Journal of Non-Crystalline Solids, 2004, 344(1-2): 1-7.
[10] NAKAMOTO M, KIYOSE A, TANAKA T, HOLAPPA L, HAMALAINEN M. Evaluation of the surface tension of ternary silicate melts containing Al2O3, CaO, FeO, MgO or MnO [J]. ISIJ International, 2007, 47(1): 38-43.
[11] NAKAMOTO M, TANAKA T, HOLAPPA L, HAMALAINEN M. Surface tension evaluation of molten silicates containing surface-active components (B2O3, CaF2 or Na2O) [J]. ISIJ International, 2007, 47(2): 211-216.
[12] HANAO M, TANAKA T, KAWAMOTO M, TAKATANI K. Evaluation of surface tension of molten slag in multi-component systems [J]. ISIJ International, 2007, 47(7): 935-939.
[13] CHOI J Y, LEE H G. Thermodynamic evaluation of the surface tension of molten CaO-SiO2-Al2O3 ternary slag [J]. ISIJ International, 2002, 42(3): 221-228.
[14] BUTLER J A V. The thermodynamics of the surfaces of solutions [J]. Proceedings of the Royal Society of London: Series A, 1932, 135: 348-375.
[15] SHANNON R D, PREWITT C T. Effective ionic radii in oxides and fluorides [J]. Acta Crystallogr B, 1969, 25(5): 925-946.
[16] SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides [J]. Acta Crystallogr A, 1976, 32: 751-767.
[17] MILLS K C, KEENE B J. Physical properties of BOS slags [J]. International Materials Reviews, 1987, 32(1-2): 1-120.
[18] IKEMIYA N, UMEMOTO J, HARA S, OGINO K. Surface tensions and densities of molten Al2O3, Ti2O3, V2O5 and Nb2O5 [J]. ISIJ International, 1993, 33(1): 156-165.
[19] RASMUSSEN J J. Surface tension, density, and volume change on melting of Al2O3 systems, Cr2O3, and Sm2O3 [J]. Journal of the American Ceramic Society, 1972, 55(6): 326-327.
RE2O3-MgO-SiO2 (RE=La, Nd, Sm, Gd和Y)熔体表面张力的计算模型
吴铖川,成国光
北京科技大学 钢铁冶金新技术国家重点实验室,北京 100083
摘 要:基于熔体组元离子半径和Butler方程,建立RE2O3-MgO-SiO2(RE=La,Nd,Sm,Gd和Y)熔体表面张力热力学计算模型。本模型利用纯组元的表面张力和摩尔体积以及熔体中各组元阳离子和阴离子半径可以获得E2O3-MgO-SiO2熔体表面张力随熔渣成分和温度的变化规律。计算1873 K La2O3-MgO-SiO2熔体等表面张力线并研究熔体成分对表面张力的影响。1873 K的纯组元La2O3,Gd2O3,Nd2O3和Y2O3的表面张力通过本模型计算分别为686、677、664和541 mN/m。除了Y2O3外,纯稀土氧化物的表面张力随其阳离子磁场强度增加而呈线性减小,而Y2O3的表面张力相对减小更多。表面张力的计算结果与文献数据一致,1873 K本模型平均偏差为1.05%。
关键词:稀土氧化物;表面张力;离子半径;Butler方程;计算模型
(Edited by Yun-bin HE)
Foundation item: Project (51374020) supported by the National Natural Science Foundation of China
Corresponding author: Cheng-chuan WU; Tel: +86-15201451151; E-mail: wuchengchuan163@163.com
DOI: 10.1016/S1003-6326(14)63517-2
Abstract: A thermodynamic model was developed for determining the surface tension of RE2O3-MgO-SiO2 (RE=La, Nd, Sm, Gd and Y) melts considering the ionic radii of the components and Butler’s equation. The temperature and composition dependence of the surface tensions in molten RE2O3-MgO-SiO2 slag systems was reproduced by the present model using surface tensions and molar volumes of pure oxides, as well as the anionic and cationic radii of the melt components. The iso-surface tension lines of La2O3-MgO-SiO2 slag melt at 1873 K were calculated and the effects of slag composition on the surface tension were also investigated. The surface tensions of La2O3, Gd2O3, Nd2O3 and Y2O3 at 1873 K were evaluated as 686, 677, 664 and 541 mN/m, respectively. The surface tension of pure rare earth oxide melts linearly decreases with increasing cationic field strength, except for Y2O3 oxide, while Y2O3 has a much weaker surface tension. The evaluated results of the surface tension show good agreements with literature data, and the mean deviation of the present model is found to be 1.05% at 1873 K.


