
Electrochemical behavior of magnesium alloys in simulated body fluids
ZENG Rong-chang(曾荣昌)1, CHEN Jun(陈君)1, W. Dietzel2, N. Hort2, K.U. Kainer2
1. School of Materials Science and Engineering, Chongqing Institute of Technology, Chongqing 400050, China;
2. GKSS Research Centre Geesthacht CmbH, Geesthacht 21502, Germany
Received 15 July 2007; accepted 10 September 2007
Abstract:
Potentiodynamic electrochemical technique was utilized to study the corrosion behavior of magnesium alloys in simulated body fluids (SBFs). The influence of materials, solutions and their temperature on corrosion rate was mainly discussed. The results demonstrate that the free corrosion potential (Ecorr) of AZ31 and AZ91 alloys rises rapidly at initial stage, and then stabilizes at some value. Ecorr of WE43 alloy increases continuously. While Ecorr of AZ91 alloy with macro-arc oxidation (MAO) coating decreases drastically in 3 min, and then fluctuates between -1 607 mV and -1 503 mV. The WE43 alloy has better corrosion resistance in Hank’s solution, compared with AZ31 and AZ91 alloys. Corrosion rates of the alloys are sensitive to the chemical composition and temperature of SBFs. A thin MgF2 film slightly improves corrosion resistance. An MAO coating on AZ91 alloy significantly reduces corrosion rate and enhances Ecorr. Pitting corrosion occurs on both AZ31 and WE43 alloys in Hank’s solution.
Key words:
magnesium alloy; biomaterial; corrosion behavior; macro arc oxidation; Hank’s solution;
1 Introduction
The study of biodegradable implant materials is one of the most interesting research topics in the forefront[1-2]. Currently, there is a huge demand in the implant markets in the world[1]. Magnesium and its alloys are potential biomaterials for their good biocompatibility and outstanding biological performance [2-3]. Unfortunately, rapid corrosion is an intrinsic response of magnesium alloys to chloride containing solution, including the human body fluid and blood plasma[4]. Moreover, release of hydrogen gas may be too quick to be tolerated by the host tissues during the degradation process[2].
It is well known that the corrosion is significantly affected by environment. For instance, the physiological environment contains various anions including phosphates, sulfates, and carbonates as well as chlorides that attack the materials. The alloys may be corroded differently. Improvement in corrosion resistance of magnesium in body fluids is an important issue in the development of magnesium implants. WITTE et al[5-7] have conducted the in-vivo and in-vitro degradation experiments of a variety of magnesium alloys as biomaterials. The in-vivo corrosion measurements demonstrate that magnesium alloys AZ31, AZ91, WE43 and LAE442 implants degrade depending on their composition of the alloying elements[5]. The previous studies, however, provide little information on the influence of solution’s composition, and their temperature and protective coating on corrosion in human body fluids. In this study, the electrochemical behavior of magnesium alloys AZ91, AZ31 and WE43 in simulated body fluids(SBFs) at 37 ℃ was investigated. The impact of coatings, such as macro-arc oxidation(MAO) on AZ91 alloy and MgF2 on AZ31 alloy, in SBF was also discussed.
2 Experimental
2.1 Materials
The materials used in this study were as-extruded AZ31 alloy (2.5%-3.0% Al, 0.7%-1.3% Zn, Mg, Bal.), as-cast WE43 alloy (3.7%-4.3%Y, 2.4%-4.4% rare earth, 0.4% Zr, Mg, Bal.) and AZ91 alloy (8.5%-9.5% Al, 0.45%-0.90% Zn, 0.17%-0.50% Mn, Mg, Bal.) with and without MAO coating, which were prepared by
Institute of Metals Research, Chinese Academy of Sciences. The film (MgF2) containing fluoride on AZ31 alloy was prepared in the HF solution for 5 min immersion.
2.2 Solution
In the study, 0.9% NaCl solution and Hank’s solution (NaCl 8.0 g/L, KCl 0.4 g/L, CaCl2 0.14 g/L, NaHCO3 0.35 g/L, C6H6O6 1.0 g/L, MgCl2?6H2O 0.1 g/L, MgSO4?7H2O 0.06 g/L, KH2PO4 0.06 g/L, Na2HPO4? 12H2O 0.06 g/L) were used as SBFs.
2.3 Electrochemical measurement
Most of the electrochemical tests were performed in SBF at (37±1)℃. The electrolyte cell was heated in a water bath. Some tests were conducted at room temperature (RT) in order to compare the influence of temperature on corrosion rate. The samples were polished to 1000 grit by using SiC papers, and then cleaned with acetone and dried with hot air. Potentiodynamic electrochemical tests were carried out by using an EG & G Model 273 potentiostat. Three- electrode system was applied: the working electrode exposed a surface area of 2.84 cm2; an saturated calomel electrode (SCE) and a platinum plate were used as reference electrode and auxiliary electrode, respectively. The polarization measurements started after the samples were immersed in the solution for 5 min. The potential scanned from -300 to 300 mV versus open current potential with a scanning rate of 0.5 mV/s.
3 Results and discussion
3.1 Potential as function of time
The free corrosion potential (Ecorr) implies that electrochemical reactions occur on the electrode-solution surface. Fig.1 shows Ecorr as a function of time for AZ31, WE43 and AZ91 alloys with and without MAO coating in Hank’s solution at 37 ℃. At the initial stage, Ecorr of AZ31, WE43 and AZ91 alloys without coating shifts to the positive direction gradually. This phenomenon is similar to what was observed on AZ80 alloy in 3.5 % NaCl solution saturated with Mg (OH)2[8] and on AZ91 alloy in SBF[9].
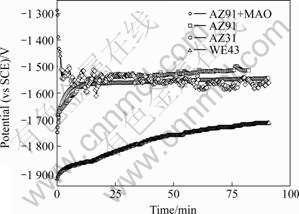
Fig.1 Ecorr as function of time for AZ31, WE43 and AZ91 alloys with and without MAO coating in Hank’s solution at 37 ℃
The previous studies[8, 10] have demonstrated that surface films on magnesium alloys, composed mainly of Mg(OH)2 , formed rapidly after immersion in the pure water, NaCl, and Na2SO4 solution. Herein, the potential of AZ31 alloy stabilizes between -1 545 and -1 564 mV after 500 s. The potential of AZ91 alloy shifts to a positive value that exceeds the potential of AZ31 alloy after 828 s and then remains steady at the level, because of higher aluminum content in the oxide of AZ91 alloy rather than in that of AZ31 alloy. It is noticeable that Ecorr vs time curve of WE43 alloy lies on the bottom of the figure. An increase in potential of WE43 alloy reaches up to 207 mV from -1 922 to -1 715 mV in 5 min. It has been reported that rare earth element could enrich on the surface, and modify the surface film of magnesium alloys[11]. For instance, the pseudo-passivation occurs in rapidly solidified Mg-Y alloys. However, the potential of AZ91 alloy with MAO decreases drastically in 180 s, and then fluctuates between -1 607 and -1 503 mV. This is caused by the fact that magnesium is easily oxidized to form a thick oxide/hydroxide film when it comes into contact with humid air or water[10]. The drop in potential in 180 s exhibits that the oxide film reacts quickly with water and forms magnesium hydroxide as shown in Eqn.(1).
MgO+H2O→Mg(OH)2 (1)
After the dissolution of MgO, magnesium substrate will continue to react with the media and magnesium hydroxide is produced and hydrogen gas evolves:
Mg+2H2O→Mg(OH) 2+H2↑ (2)
The big undulation of AZ91 alloy with MAO in Ecorr reflects the formation and breakdown of partially protective film Mg (OH)2.
3.2 Polarization curves
3.2.1 Influence of materials
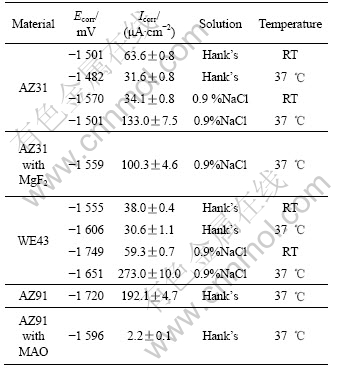
The polarization curves of AZ31, AZ91 and WE43 alloys in SBF at 37 ℃ are shown in Fig.2.
Table 1 lists the electrochemical parameters. It is obvious that the corrosion rate of WE43 alloy is approximately equal to that of AZ31 alloy and is significantly lower than that of AZ91 alloy in Hank’s solution.
NAKATSUGAWA et al[12] have reported that the addition of heavy rare earth elements is effective in suppressing Mg corrosion. Maybe the corrosion rate of the WE43 alloy can be slightly reduced by the rare earth element Y. However, the corrosion rate of WE43 alloy is twice higher than that of AZ31 alloy in 0.9 % NaCl solution at 37 ℃, although an increase in aluminum could lead to a decreased corrosion rate[12]. Herein, other factors such as the microstructure grain size and stress may affect the corrosion behavior of these alloys. It is noteworthy that a breakdown potential (Eb), indicating the tendency to localized corrosion, exists in the curve of AZ91 alloy. It is postulated that the WE43 alloy containing rare earth element may be one of the promising implant materials from the point of corrosion views.

Fig.2 Polarization plots of AZ31, AZ91 and WE43 alloys in SBF at 37 ℃
Table 1 Electrochemical parameters of alloys in SBFs
3.2.2 Influence of solutions and their temperature
Fig.3 exhibits the polarization curves of AZ31 and WE43 alloys in Hank’s solution at ambient temperature and 37 ℃. The electrochemical parameters of the alloys are also listed in Table 1. It also reveals that the corrosion rate of magnesium alloys AZ31 and WE43 in Hank’s solution is remarkably lower than that in 0.9% NaCl solution at 37 ℃.
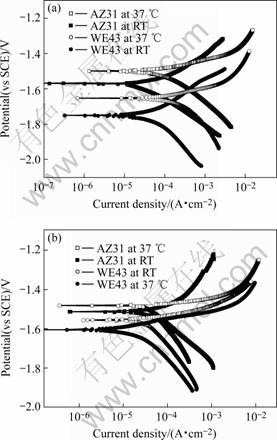
Fig.3 Polarization curves of AZ31 and WE43 alloys in 0.9% NaCl solution (a) and Hank’s solution (b) at ambient temperature and 37 ℃
It is evident that the corrosion rates of the alloys are very sensitive to temperature of the solution. In 0.9 % NaCl solution, corrosion rates of AZ31 and WE43 alloys increase from (34.1±0.8) μA/cm2, (59.3±0.7) μA/cm2 to (133.0±7.5) μA/cm2, (273.0±10.0) μA/cm2 with the ambient temperature shifting to 37 ℃, respectively. Whereas, in Hank’s solution, the corrosion rates of AZ31 and WE43 alloys decrease from (63.6±0.8) μA/cm2 and (38.0±0.4) μA/cm2 to (31.6±0.8) μA/cm2 and (30.6±1.1) μA/cm2 with the temperature increasing from ambient temperature to 37 ℃. This unexpected phenomenon may be associated with the influence of ions like Ca2+, ![]() ,
, ![]() and
and ![]() , which might to some degree suppress the detrimental impact of Cl- ion[13].
, which might to some degree suppress the detrimental impact of Cl- ion[13].
3.2.3 Influence of protective coating
Figs.4 (a) and (b) show the polarization curves of AZ31 alloy with and without MgF2 film in 0.9% NaCl solution and AZ91 alloy with and without MAO coating in Hank’s solution at 37 ℃, respectively. Their electrochemical parameters are listed in Table 1, too.
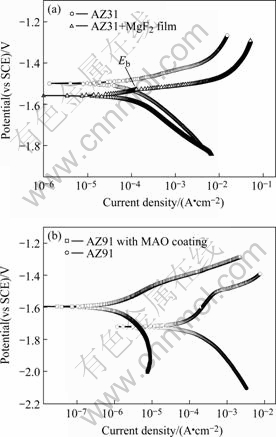
Fig.4 Polarization curves of (a)AZ31 alloy with and without MgF2 film in 0.9% NaCl solution and (b) AZ91 alloy with and without MAO coating in Hank’s solution at 37 ℃
It is apparent that the thin MgF2 film on AZ31 alloy slightly improves the corrosion resistance in 0.9 % NaCl solution. And the MAO coating on magnesium alloy AZ91 significantly enhances the potential and reduces the corrosion rate in Hank’s solution at 37 ℃. The results indicate that a suitable coating can lead to a decrease in corrosion rate of magnesium alloys in SBFs.
3.3 Corrosion morphologies
The corrosion pits on AZ31 and WE43 alloys after 1 h immersion in Hank’s solution at 37 ℃ are observed, as shown in Figs.5 (a) and (b), respectively. The number of pits increases gradually with the immersion time. A layer of white insoluble corrosion product Mg (OH)2 is deposited on the surfaces Figs.5.

Fig.5 Corrosion morphologies for (a)AZ31 alloy and (b) WE43 alloy after 1 h immersion in Hank’s solution at 37 ℃
4 Conclusions
1) Ecorr of the alloys (AZ31 and AZ91) containing aluminium increases rapidly at initial stage, and then stabilizes at some value. While Ecorr of WE43 alloy containing rare earth increases continuously. Compared with AZ31 and AZ91 alloys, WE43 alloy has better corrosion resistance in Hank’s solution.
2) The corrosion rates of magnesium alloys are sensitive to the chemical composition and temperature of solutions. In 0.9% NaCl solution, corrosion rates of AZ31 and WE43 alloys increase from (34.1±0.8) μA/cm2, (59.3±0.7) μA/cm2 to (133.0±7.5) μA/cm2, (273.0±10.0) μA/cm2 with the ambient temperature shifting to 37 ℃, respectively. Whereas, in Hank’s solution, corrosion rates of AZ31 and WE43 alloys decrease from (63.6±0.8) μA/cm2 and (38.0±0.4) μA/cm2 to (31.6±0.8) μA/cm2 and (30.6±1.1) μA/cm2 with the temperature increasing from ambient temperature to 37 ℃.
3) A thin MgF2 film slightly improves corrosion resistance; the macro-arc oxidation (MAO) coating on AZ91 alloy significantly reduces the corrosion rate and enhances free corrosion potential.
Acknowledgement
Thanks go to Guangling Magnesium Industry Science and Technology Co. Ltd. for providing the tested materials and Dr. ZHANG Rong-fa for offering the samples of AZ91 alloy with MAO.
References
[1] MARREY R V, BURGERMEISTER R, GRISHABER R B, RITCHIE R O. Fatigue and life for cobalt-chromium stents: A fracture mechanics analysis [J]. Biomaterials, 2006, 27: 1988-2000.
[2] STAIGER M P, PIETAK A M, HUADMAI J, DIAS G. Magnesium and its alloys as orthopedic biomaterials: A review [J]. Biomaterials, 2006, 27: 1728-1734.
[3] SARIS N L, MERVAALA E, KARPPANEN H, KHAWAJA J A, LEWENSTAM A. Magnesium: An update on physiological, clinical and analytical aspects [J]. Clinica Chimica Acta, 2000, 294: 1-26.
[4] SONG G. Control of biodegradation of biocompatible magnesium alloys [J]. Corros Sci, 2007, 49: 1696-1701.
[5] WITTE F, KAESE V, HAFERKAMP H, SWITZER E, MEYER-LINDENBERG A, WIRTH C J, WINDHAGEN H. In-vivo corrosion of four magnesium alloys and the associated bone response [J]. Biomaterials, 2005, 26: 3557-3563.
[6] WITTE F, FISCHER J, NELLESEN J, CROSTACK H A, KAESE V, PISCH A, BECKAMANN F, WINDHAGEN H. In vitro and in vivo corrosion measurements of magnesium alloys [J]. Biomaterials, 2006, 27: 1013-1018.
[7] WITTE F, REIFENRATH J, MUELLER P P, CROSTACK H A, NELLESEN J, BACH F W, BORMANN D, RUDERT M. Cartilage repair on magnesium scaffolds used as a subchondral bone replacement [J]. mat wiss u Werkstofftech, 2006, 37(6): 504-508.
[8] ZENG Rong-chang, HAN En-hou, KE Wei. Corrosion of artificial aged magnesium alloy AZ80 in 3.5% NaCl solution [J]. J Mater Sci Technol, 2007, 23(3): 353-358.
[9] XIN Y, LIU C, TANG G, TIAN X, CHU P K. Corrosion behavior of biomedical AZ91 magnesium alloy in simulated body fluids [J]. J Mater Res, 2007, 22(7): 2004-2011.
[10] HARA N, KOBAYASHI Y, KAGAYA D, AKAO N. Formation and breakdown of surface films on magnesium and its alloys in aqueous solutions [J]. Corros Sci, 2007, 49: 166-175.
[11] YAMASAKI M, HAYASHI N, IZUMI S, KAWAMURA Y. Corrosion behavior of rapidly solidified Mg-Zn-rare earth element alloys in NaCl solution [J]. Corros Sci, 2007, 49: 255-262.
[12] NAKATSUGAWA I, TAKAYASU H, ARAKI K, TSUKEDA T. Electrochemical corrosion studies of thixomolded AZ91D in sodium chloride solution [J]. Mater Sci Forum, 2003, 419/422: 845-850.
[13] SONG G, ATRENS A, St. JOHN D, WU X, NAIM J. The anodic dissolution of magnesium in chloride and sulphate solutions [J]. Corros Sci, 1997, 39: 1981-2004.
Foundation item: Project (8655) supported by the Natural Science Foundation of Chongqing Municipal Science and Technology commission, China; Project(50405005) supported by the National Science Foundation of China
Corresponding author: ZENG Rong-chang; Tel: +86-23-68665616; E-mail: rczeng2001@yahoo.com.cn
(Edited by CHEN Wei-ping)


