
ARTICLE
J. Cent. South Univ. (2019) 26: 1540-1549
DOI: https://doi.org/10.1007/s11771-019-4110-y
Hydrogen reduced sodium vanadate nanowire arrays as electrode material of lithium-ion battery
XU Xue-liu(徐学留)1, LI Guang-zhong(李广忠)2, FU Ze-wei(符泽卫)3, HU Jun-tao(胡俊涛)3,
LUO Zhi-ping(罗治平)4, HUA Kang(华康)1, LU Xue-qin(陆雪芹)5,FANG Dong(方东)1, BAO Rui(鲍瑞)1, YI Jian-hong(易健宏)1
1. Faculty of Materials Science and Engineering, Kunming University of Science and Technology,Kunming 650093, China;
2. State Key Laboratory of Porous Metal Materials, Northwest Institute for Nonferrous Metal Research,Xi’an 710016, China;
3. Yunnan Tin Group (Holding) Co. Ltd, Kunming 650000, China;
4. Department of Chemistry and Physics, Fayetteville State University, Fayetteville, NC 28301, USA;
5. School of Foreign Languages, Wuhan Textile University, Wuhan 430073, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
Vanadates and vanadium oxides are potential lithium-ion electrode materials because of their easy preparation and high capacity properties. This paper reports the electrochemical lithium-storage performance of VO2 and NaV2O5 composite nanowire arrays. Firstly, Na5V12O32 nanowire arrays are fabricated by a hydrothermal method, and then VO2 and NaV2O5 composite nanowire arrays are prepared by a reduction reaction of Na5V12O32 nanowire arrays in hydrogen atmosphere. Crystal structure, chemical composition and morphology of the prepared samples are characterized in detail. The obtained composite is used as an electrode of a lithium-ion battery, which exhibits high reversible capacity and good cycle stability. The composite obtained at 500 °C presents a specific discharge capacity up to 345.1 mA·h/g after 50 cycles at a current density of 30 mA/g.
Key words:
sodium vanadate; hydrogen reduction; nanowire array; lithium-ion battery;
Cite this article as:
XU Xue-liu, LI Guang-zhong, FU Ze-wei, HU Jun-tao, LUO Zhi-ping, HUA Kang, LU Xue-qin, FANG Dong, BAO Rui, YI Jian-hong. Hydrogen reduced sodium vanadate nanowire arrays as electrode material of lithium-ion battery. [J]. Journal of Central South University, 2019, 26(6): 1540-1549.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4110-y1 Introduction
Today, lithium-ion batteries have been widely used in daily life in various fields, such as mobile phones, notebook computers, and electric vehicles. Lithium-ion batteries are limited in their applications and performance due to their limitations in energy density, cycle stability and rate performance. Therefore, it is necessary to develop new electrode materials to optimize the overall electrochemical performance of the battery system. With the advantages of high working voltage, high specific capacity, stable discharge performance and wide operating temperature range, etc., vanadates and vanadium oxides are the potential candidates of electrode materials [1-5]. In addition, vanadium is a widely distributed trace element on the earth, accounting for 0.02% of the earth’s crust. Rich reserves can effectively reduce the cost of the electrode material. There are four valence states of vanadium element, which are V2+, V3+, V4+ and V5+. The variable chemical state allows vanadium element to form different vanadium oxides or vanadates.
Up to now, vanadium oxides and vanadates have attracted more and more scientists’ attention as electrode materials for batteries [6, 7]. CAO et al [8] synthesized Na5V12O32 nanowire arrays by a hydrothermal method on a titanium foil as an electrode material. The electrode material exhibited significant capacity stability over 50 cycles at a rate of 50 mA/g, with a capacity of 289.7 mA·h/g. BALOGUN et al [9] designed a novel independent carbon quantum dot coated VO2 interlaced nanowires by a simple manufacturing process. The VO2 electrodes modified by carbon quantum dots have lithium and sodium storage capacities of 420 and 328 mA·h/g, respectively, at a fixed rate of 0.3C. The V3O7·H2O nanobelts have a high initial discharge specific capacity of 253.0 mA·h/g in the potential range of 3.8-1.7 V, and its stable capacity still retains 228.6 mA·h/g after 50 cycles [10]. V2O5 nanoribbon arrays on conductive substrates present an enhanced electrochemical performance in lithium-ion battery [11]. CAO et al [12] made a novel sodium vanadate complex (Na5V12O32@PPy) nanowire by ordinary hydrothermal and electrodeposition methods, and the compound has a synergistic core-shell structure. LU et al [13] successfully prepared Na0.33V2O5 nanosheet-graphene hybrids using a hydrothermal and freeze-drying technique. This electrode material can maintain a high discharge capacity of 199 mA·h/g even after 400 cycles at a very high current density of 4.5 A/g. The average fading rate per cycle is 0.03%. Cobalt vanadate (CoV3O8) shows nearly 100% coulombic efficiency in 100 discharge-charge cycles, showing a reversible capacity of approximately 120 mA·h/g between 1.5 and 4.5 V [14]. LEI et al [15] used V2O5 as a precursor material to prepare MnV2O6 nanowire materials by a hydrothermal method and the initial discharge capacity can reach 650 mA·h/g. It can be found that most of the current literatures focus on a single component, or in combination with a conductive agent, as the lithium-ion battery electrode. There is no research report on vanadate and vanadium oxide composite electrodes [16-21].
This work reports VO2 and NaV2O5 composite nanowire arrays for lithium-ion battery, which is synthesized by a reduction of Na5V12O32 nanowire arrays at 500 °C for 5 h in 5% H2 and 95% Ar mixed atmosphere. The structure, crystal form, composition, and lithium-storage performance, etc., of the samples are characterized and presented in detail.
2 Material and method
2.1 Synthesis of electrode materials
Na5V12O32 nanowires were obtained by a hydrothermal method using Ti foil as substrate. A mixed aqueous solution was prepared with hexamethylenetetramine: NH4VO3 (0.28 mol/L): NaCl: H2C2O4·2H2O in the ratio of 0.2:1:6:2 added into 45 mL deionized water [22]. A polished and water-washed titanium sheet (99.5% purity) was placed in the reaction vessel. The vessel was then sealed in an autoclave and transferred to an electric furnace at 150 °C for 60 min. After naturally cooled to room temperature, the titanium substrate was taken out from the reaction vessel and washed several times with deionized water and pure ethanol, and then dried in an oven for 24 h. Subsequently, the dried sample was calcined at 300, 500 and 800 °C in a mixed atmosphere containing 5% H2 and 95% Ar for 5 h, respectively.
2.2 Characterization
The crystal structure of the samples was characterized with an X-ray diffractometer (Bruker D8 XRD), and the morphology of the samples was characterized by using scanning electron microscope (SEM) (Hitachi S-4800 and JEOL-300) with an energy-dispersive spectrometer (EDS), and transmission electron microscope (TEM) (JEOL- 2100F at 200 kV). Analysis of chemical composition of samples was done by X-ray photoelectron spectroscopy (XPS, Kα 1063, Termo Fisher Scientifc, UK).
2.3 Electrochemical measurements
Electrochemical testing of lithium-ion batteries was performed using a CR2016 button battery. Half-cell electrolyte was prepared using 1 mol/L LiPF6 solution (ethylene carbonate (EC): ethyl methyl carbonate (EMC): dimethyl carbonate (DMC)=1:1:1, in volume ratio), and Li metal was used as the counter electrode. The samples reduced by hydrogen at 300 and 500 °C were assembled as working electrodes of the batteries in a glovebox (Mbraun, Lab Master 100, Germany) containing argon atmosphere. The volt-ampere cycle curve (CV) was measured on an electrochemical workstation (Corr Test CS350), and the operating voltage was 0.01 to 3 V vs. Li/Li+ with a scan rate of 0.3 mV/s. Constant current charge-discharge measurement was done on a NEWARE battery tester (CT 3008). All tests were measured at 25 °C.
3 Results and discussion
The crystal structure of the product at different temperatures (300, 500 °C) was analyzed by XRD, as depicted in Figure 1. The sample calcined at 300 °C has a crystal structure similar to that of the Na5V12O32 precursor with low crystallinity [23]. It can be seen that the sample calcined at 500 °C has higher diffraction peaks which are from the tetragonal phase [space group: P42/mm (136) Z=2] of VO2 (PDF card No. 76-0675 marked by ▼), with lattice parameters of a=4.5561  , c=2.8598
, c=2.8598  ; and an orthorhombic crystalline phase [space group: P21mn (31) Z=2] of NaV2O5 (PDF card No. 70-0870 marked by·), with lattice parameters of a=11.318
; and an orthorhombic crystalline phase [space group: P21mn (31) Z=2] of NaV2O5 (PDF card No. 70-0870 marked by·), with lattice parameters of a=11.318  , b=3.611
, b=3.611  , c=4.797
, c=4.797  .
.

Figure 1 XRD patterns of as-prepared precursor and samples annealed at different temperatures
The mass fractions of VO2 and NaV2O5 were calculated by the following formula (1). According to Jade software analysis: Ka(=RIR (VO2)) is 3.77, Kb(=RIR (NaV2O5)) is 1.59, where RIR is the reference intensity ratio. According to the formula, the quality scores of VO2 and NaV2O5 are 22.7% and 77.3%, respectively [24].
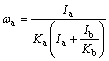 (1)
(1)
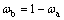 (2)
(2)
where K is RIR value; I is the highest peak intensity; ω is phase quality score.
Morphology of products was studied with SEM. The as-prepared precursor was annealed at 300 °C in 5% H2 and 95% Ar mixed atmosphere Figure 2 reveals that the nanowire arrays are presented vertically on the surface of the Ti foil with a similar structure of the Na5V12O32 nanowire(precursor) as shown in Figure 3. The morphological characteristics of the as-prepared precursor annealed at 300 °C are further studied with TEM, as shown in Figures 2 (c) and (d). The nanowires are about 50 nm in diameter and about several microns in length. In Figure 2(d), the lattice fringe spacing is about 0.21 nm, which is from the plane (102) of NaV2O5. The EDS result is shown in Figure 2(e), where Na, V, and O elements are presented. At the same time, the Ti element can also be observed from the spectrum, which is derived from the Ti substrate.
Further, the samples annealed at 500 °C were tested with SEM and TEM as shown in Figure 4. The dark green nanowire arrays are vertically on the surface of the Ti foil, as shown in Figure 4(a). In Figure 4(b), the diameter of the nanowires is about 80 nm, which is consistent with the SEM test result (Figure 4(a)). The lattice fringe spacing in Figure 4(c) is 0.21 nm, which originates from the plane (102) of NaV2O5. The detailed structure of the as-prepared precursor annealed at 800 °C was also examined with SEM as shown in Figure 4(d), while the sample was partially melted and destroyed.
In order to further study the surface information of the precursor after calcination at 500 °C, XPS analysis is conducted, as shown in Figure 5. A survey XPS spectrum (Figure 5(a)) has four distinct peaks at 1071.8, 529.62, 514.57 and 282.5 eV, which can be assigned to Na 1s, O 1s,V 2p and C 1s, respectively. The peak at 284.6 eV is the C 1s peak of the calibrated reference binding energy. The carbon peak appearing in the sample may be from the CO2 in air. Figure 5(b) exhibits a higher solution XPS of V 2p. The peaks belong to V5+ 2p3/2 and V5+ 2p1/2 states appearing at 517.66 and 524.90 eV, respectively. There are two more peaks at 516.66 and 523.80 eV, which are attributed to V4+ 2p3/2 and V4+ 2p1/2 states [8], respectively. In Figure 5(c), it can be clearly observed that the peaks of the XPS spectrum of O 1s are at 530.84 and 531.96 eV, respectively, which may be due to the formation of M—O (M=Na, V) and C—O species, respectively [25]. The peak at 1071.8 eV in Figure 5(d) is from Na 1s.
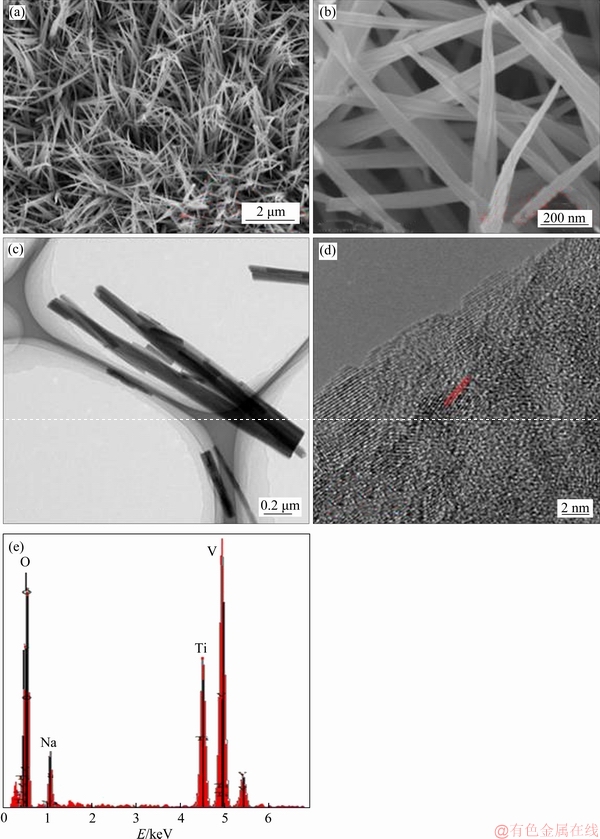
Figure 2 SEM images (a, b), TEM images (c, d) and EDS (e) of as- prepared product annealed at 300 °C
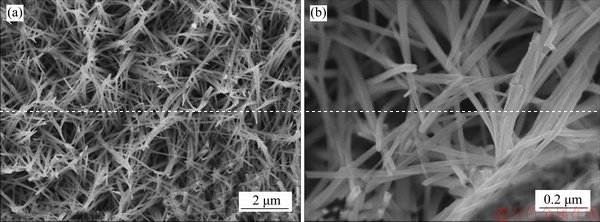
Figure 3 SEM images of as-prepared Na5V12O32 nanowires (precursor) on Ti foil

Figure 4 SEM images (a), TEM image (b) and HR-TEM image (c) of as-prepared precursor annealed at 500 °C, and SEM images of as-prepared precursor annealed at 800 °C (d)(The inset images in (a) and (d) are the corresponding low magnification figure)
The electrochemical properties of VO2 and NaV2O5 composite nanowire arrays obtained at 500 °C as lithium-ion storage electrode were tested as shown in Figure 6. Figure 6(a) depicts the first three discharge/charge plots at a current density of 30 mA/g. The discharge capacities are 558.0, 529.5 and 487.9 mA·h/g at the 1st, 2nd and 3rd cycles, respectively, indicating a gradual decay in the first three cycles. At a current density of 30 mA/g, the cycle performance of the sample was examined in a voltage range of 0.01-3.0 V (vs. Li/Li+), as shown in Figure 6(b). After 50 cycles, the discharge-charge capacity remains near a straight line, indicating that the battery capacity is stable. Further, the rate performance at different current densities was tested, ranging from 30 to 300 mA/g, with a potential range of 0.01-3.0 V, as shown in Figure 6(c). The specific discharge capacities of the electrodes at current densities of 30, 50, 100, 200 and 300 mA/g were 585.4, 449.1, 296.5, 201.7 and 156.4 mA·h/g, respectively. When cycling at 30 mA/g again, the discharge capacity returned to 357.2 mA·h/g.Figure 6(d) presents the capacity retentions after 50 cycles of the sample obtained by calcination at 300 °C, which shows that the electrochemical performance is lower than that of the sample obtained at 500 °C. The VO2 and NaV2O5 composite electrode has a better electrochemical performance than the independent VO2 [25] or NaV2O5 [26] electrodes, which may be caused by some reasons as follows. The nano-three- dimensional electrode can increase the electrode/ electrolyte contact areas and shorten the lithium-ion diffusion pathways, potentially increasing the rate performance. VO2 [25] or NaV2O5 [26] has different lithium-ion insertion- extraction potentials, which make the electrode materials have a certain complementarity during charge and discharge process. It can alleviate stress concentration and improve electrode stability.
VO2+xLi++xe- LixVO2 (3)
LixVO2 (3)
NaV2O5+xLi++xe- LixNaV2O5<> (4)
LixNaV2O5<> (4)
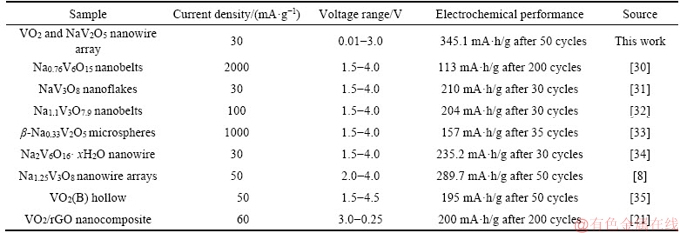
The XPS spectra analyses of VO2 and NaV2O5 composite nanowire arrays discharged to 0.01 V after 50 cycles were further performed to test the valence of the key elements (V and O elements). Before the XPS test, in order to eliminate the effects of solid electrolyte interface (SEI) film, an Ar+ beam was used to etch the sample. The binding energies of O are 530.24, 530.77, 531.58 and 533.06 eV in Figure 7(a), which are attributed to the M—O (M=Na, V), water or moisture, C—OH and C=O species, respectively [27]. The oxidation state of V is fitted and the binding energies of V2+, V3+, V4+ and V5+ 2p3/2 are 513.85, 515.05, 515.98 and 516.95 eV, respectively, as shown in Figure 7(b) [28]. The low oxidation states of the V element (such as V2+ and V3+) appear in Figure 7(b), indicating that redox reactions of V occur during charge-discharge process [29]. According to the XPS results, the theoretical capacities of the materials are calculated as listed in Tables 1 and 2. Further, a performance comparison with the vanadium-containing electrodes reported in the literatures is shown in Table 3. The VO2 and NaV2O5 composite has a relatively high lithium storage capacity.

Figure 5 XPS of as-prepared precursor annealed at 500 °C:
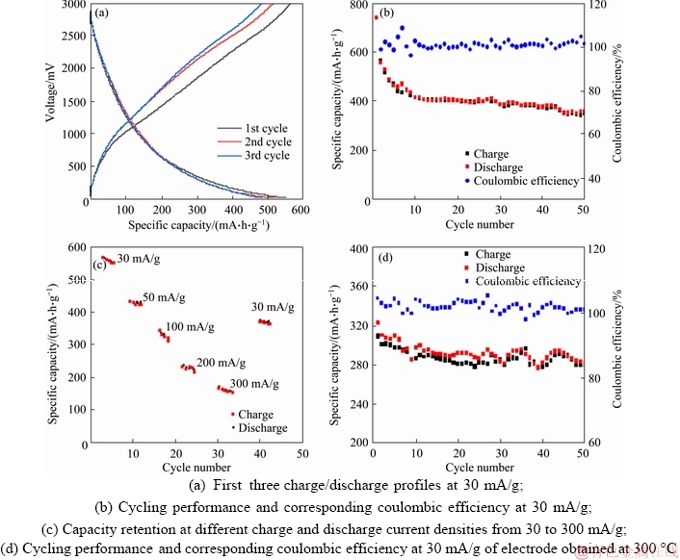
Figure 6 Electrochemical performance of VO2 and NaV2O5 composite nanowire arrays (500 °C):

Figure 7 XPS spectra of VO2 and NaV2O5 composite nanowire arrays discharged to 0.01 V:
Table 1 Theoretical capacity of vanadium in different valence states (NaV2O5)
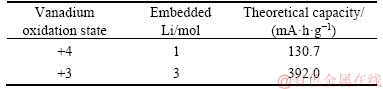
Table 2 Theoretical capacity of vanadium in +3 valence state (VO2)

Table 3 Electrochemical properties of previously reported lithium vanadate for lithium-ion battery
4 Conclusions
VO2 and NaV2O5 composite nanowire arrays are successfully prepared by a reduction reaction of Na5V12O32 nanowire arrays in hydrogen atmosphere. Samples annealed at 300 and 500 °C retain their original appearance, presenting nanowire arrays vertically grown on the surface of the Ti foil, while the nanowires partially melt after calcination at 800 °C. Experimental studies have shown that the VO2 and NaV2O5 composite nanowire arrays obtained at 500 °C manifest good electrochemical properties for LIBs in terms of high specific capacity, remarkable cycling ability, and good rate capability in the potential range of 0.01-3.0 V. Therefore, VO2 and NaV2O5 composite nanowire arrays have potention to be used as a new electrode material for lithium-ion batteries.
References
[1] LIU Cai-ling, LUO Shao-hua, HUANG Hong-bo, WANG Zhi-yuan, WANG Qing, ZHANG Ya-hui, LIU Yan-guo, ZHAI Yu-chun, WANG Zhao-wen. Potassium vanadate K0.23V2O5, as anode materials for lithium-ion and potassium-ion batteries [J]. Journal of Power Sources, 2018, 389: 77-83.
[2] WU Hua, CUI Yi. Designing nanostructured Si anodes for high energy lithium-ion batteries [J]. Nano Today, 2012, 7(5): 414-429.
[3] XIE Xiu-qiang, SU Da-wei, SUN Bing, ZHANG Jin-qiang, WANG Cheng-yin, WANG Guo-xiu. Synthesis of single-crystalline spinel LiMn2O4 nanorods for lithium-ion batteries with high rate capability and long cycle life [J]. Chemistry-A European Journal, 2014, 20(51): 17125-17131.
[4] OHTA S, KOBAYASHI T, SEKI J, ASAOKA T. Electrochemical performance of an all-solid-state lithium-ion battery with garnet-type oxide electrolyte [J]. Journal of Power Sources, 2012, 202: 332-335.
[5] XU Jun-yi, LI Jie, ZHU Yue-wu, ZHU Kai, LIUYe-xiang, LIU Jin. A triPEG-boron based electrolyte membrane for wide temperature lithium-ion batteries [J]. Rsc Advances, 2016, 6(24): 20343-20348.
[6] MAI Li-qiang, XU Lin, HAN Chun-hua, XU Xu, LUO Yan-zhu, ZHAO Shi-yong, ZHAO Yun-long. Electrospun ultralong hierarchical vanadium oxide nanowires with high performance for lithium-ion batteries [J]. Nano letters, 2010, 10(11): 4750-4755.
[7] MAI Li-qiang, XU Xu, XU Lin, HAN Chun-hua, LUO Yan-zhu. Vanadium oxide nanowires for Li-ion batteries [J]. Journal of Materials Research, 2011, 26(17): 2175-2185.
[8] CAO Yun-he, FANG Dong, WANG Chang, LI Li-cheng, XU Wei-lin, LUO Zhi-ping, LIU Xiao-qing, XIONG Chuan-xi, LIU Su-qin. Novel aligned sodium vanadate nanowire arrays for high performance lithium-ion battery electrodes [J]. RSC Advances, 2015, 5(53): 42955-42960.
[9] BALOGUN M S, LUO Yang, LYU Fei-yi, WANG Fu-xin, YANG Hao, LI Hai-bo, LIANG Chao-lun, HUANG Miao, HUANG Yong-chao, TONG Ye-xiang. Carbon quantum dot surface-engineered VO2 interwoven nanowires: A flexible cathode material for lithium and sodium ion batteries [J]. ACS Applied Materials & Interfaces, 2016, 8(15): 9733-9744.
[10] QIAO Hui, ZHU Xian-jun, ZHENG Zhi, LIU Li, ZHANG Li-zhi. Synthesis of V3O7·H2O nanobelts as cathode materials for lithium-ion batteries [J]. Electrochemistry communications, 2006, 8(1): 21-26.
[11] WANG Yu, ZHANG Hui-juan, LI Wei-xiang, LIN Jian-yi, WONG C. Designed strategy to fabricate a patterned V2O5 nanobelt array as a superior electrode for Li-ion batteries [J]. Journal of Materials Chemistry, 2011, 21(7): 2362-2368.
[12] CAO Yun-he, FANG Dong, LIU Xiao-qing, LUO Zhi-ping, LI Guang-zhong, XU Wei-lin, JIANG Ming, XIONG Chuan-xi. Sodium vanadate nanowires @ polypyrrole with synergetic core-shell structure for enhanced reversible sodium-ion storage [J]. Composites Science and Technology, 2016, 137: 130-137.
[13] LU Ya-kun, WU Jun, LIU Jun, LEI Ming, TANG Sha-sha, LU Pei-jie, YANG Lin-yu, YANG Hao-ran, YANG Qian. Facile synthesis of Na0.33V2O5 nanosheet-graphene hybrids as ultrahigh performance cathode materials for lithium-ion batteries [J]. ACS applied materials & interfaces, 2015, 7(31): 17433-17440.
[14] HIBINO M, OZAWA N, MURAKAMI T, NAKAMURA M, YAO T. Lithium insertion and extraction of cobalt vanadium de[J]. Electrochemical and Solid-State Letters, 2005, 8(10): A500-A503.
[15] LEI Shui-jin, TANG Kai-bin, JIN Yi, CHEN Chun-hua. Preparation of aligned MnV2O6 nanorods and their anodic performance for lithium secondary battery use [J]. Nanotechnology, 2007, 18, 175605.
[16] WANG Jiang-yan, YANG Nai-liang, TANG Hong-jie, DONG Zheng-hong, JIN Quan, YANG Mei, KISAILUS D-, ZHAO Hui-jun, TANG Zhi-yong, WANG Dan. Accurate control of multishelled Co3O4 hollow microspheres as high- performance anode materials in lithium-ion batteries [J]. Angewandte Chemie International Edition, 2013, 52(25): 6417-6420.
[17] HE Zhang-xing, LI Man-man, LI Yue-hua, LI Chuan-chang, YI Zao, ZHU Jing, DAI Lei, MENG Wei, ZHOU Hui-zhu, WANG Ling. ZrO2 nanoparticle embedded carbon nanofibers by elecrospinning techniique as advanced negative electrode materials for vanadium redox flow battery [J]. Electrochimica Acta, 2019, 309: 166-176.
[18] HUAN Chang-meng, ZHAO Xin-yue, XIAO Xiu-di, LU Yuan, QI Shuai, ZHAN Yong-jun, ZHANG Ling-zhi, XU Gang. One-step solvothermal synthesis of V2O3@C nanoparticles as anode materials for lithium-ion battery [J]. Journal of Alloys and Compounds, 2019, 776: 568-574.
[19] LU Yan-rong, ZHANG Lu, CHENG Gang, WANG Peng-fei, ZHANG Tian-ze, LI Chuan-chan, JIANG Ying-qiao, HE Zhang-xing, DAI Lei, WANG Ling. Preparation of carbon nanosheet by molten salt route and its application in catalyzing VO2+/VO2+ redox reaction, ZrO2 nanoparticle embedded carbon nanofibers by electrospinning technique as advanced negative electrode materials for vanadium redox flow battery [J]. Journal of The Electrochemical Society, 2019, 166: A953-A959.
[20] LUBKE M, NING D, POWELL M J, BRETT D J L, SHEARING P R, LIU Z, DARR J A. VO2 nano-sheet negative electrodes for lithium-ion batteries [J]. Electrochemistry Communications, 2016, 64: 56-60.
[21] HE G, LI L, MANTHIRAM A. VO2/rGO nanorods as a potential anode for sodium-and lithium-ion batteries [J]. Journal of Materials Chemistry A, 2015, 3(28): 14750-14758.
[22] HUA Kang, LI Xiu-juan, BAO Rui, FANG Dong, JIANG Ming, YI Jian-hong, LUO Zhi-ping, SHU Yong-chun, SUN Ben-shuang. Electrochemical performance of silver vanadate/silver nanowire composite for lithium-ion batteries [J]. Solid State Ionics, 2018, 325: 133-140.
[23] HUA Kang, LI Xiu-juan, FANG Dong, YI Jian-hong, BAO Rui, LUO Zhi-ping. Electrodeposition of high-density lithium vanadate nanowires for lithium-ion battery [J]. Applied Surface Science, 2018, 447: 610-616.
[24] WANG Chang, CAO Yun-he, LUO Zhi-ping, LI Guang-zhong, XU Wei-lin, XIONG Chuan-xi, HE Guo-qiu, WANG Ying-de, LI Shan, LIU Hui, FANG Dong Flexible potassium vanadate nanowires on Ti fabric as a binder-free cathode for high-performance advanced lithium-ion battery [J]. Chemical Engineering Journal, 2017, 307: 382-388.
[25] PEI Cun-yuan, XIONG Fang-yu, SHENG Jin-zhi, YIN Ya-meng, TAN Shuang-shuang, WANG Dan-dan, HAN Chun-hua, AN Qin-you, MAI Li-qiang. VO2 Nanoflakes as the cathode material of hybrid magnesium-lithium-ion batteries with high energy density [J]. ACS applied materials & interfaces, 2017, 9(20): 17060-17066.
[26] BADDOUR-HADJEAN R, HUYNH L T N, EMERY N, PEREIRA-RAMOS J P. Lithium insertion in α′-NaV2O5:Na-pillaring effect on the structural and electrochemical properties [J]. Electrochemical Acta, 2018, 270: 224-235.
[27] LUKATSKAYA M, DUNN B, GOGOTSI Y. Multidimensional materials and device architectures for future hybrid energy storage [J]. Nature Communications, 2016, 7: 12647-12661.
[28] HE Han-na, SHANG Zhen-gang, HUANG Xiao-bing, TAN Shuai, SUN Dan, XU Guo-qing, TANG You-gen, WANG Hai-yan. Ultrathin (NH4)0.5V2O5 nanosheets as a stable anode for aqueous lithium-ion battery [J]. Journal of the Electrochemical Society, 2016, 163(10): A2349-A2355.
[29] HU Wen, ZHANG Xin-bo, CHENG Yong-lian, WU Yao-ming, WANG Li-min. Low-cost and facile one-pot synthesis of pure single-crystalline ε-Cu0.95 V2O5 nanoribbons: High capacity cathode material for rechargeable Li-ion batteries [J]. Chemical Communications, 2011, 47(18): 5250-5252.
[30] YANG Kai-wen, FANG Guo-zhao,ZHOU Jiang, QIN Mu-lan, TANG Yan-an, PAN An-qiang, LIANG Shu-quan. Hydrothermal synthesis of sodium vanadate nanobelts as high-performance cathode materials for lithium batteries [J]. Journal of Power Sources, 2016, 325: 383-390.
[31] TANG You-gen, SUN Dan, WANG Hai-yan, HUANG Xiao-bing, ZHANG Hui, LIU Su-qin, LIU You-nian. Synthesis and electrochemical properties of NaV3O8 nanoflakes as high-performance cathode for Li-ion battery [J]. RSC Advances, 2014, 4(16): 8328-8334.
[32] LIANG Shu-quan, ZHOU Jiang, FANG Guo-zhao, LIU Jing, TANG Yan, LI Xi-lin, PAN An-qiang. Ultrathin Na1.1V3O7.9 nanobelts with superior performance as cathode materials for lithium-ion batteries [J]. ACS Applied Materials & Interfaces, 2013, 5(17): 8704-8709.
[33] TAN Qin-guang, ZHU Qin-yu, PAN An-qiang, WANG Ya-ping, TANG Yan, TAN Xiao-ping, LIANG Shu-quan, CAO Guo-zhong. Template-free synthesis of β-Na0.33V2O5 microspheres as cathode materials for lithium-ion batteries [J]. Cryst Eng Comm, 2015, 17(26): 4774-4780.
[34] WANG Hai-yan, WANG Wen-jie, REN Yu, HUANG Ke-long, LIU Su-qin. A new cathode material Na2V6O16·xH2O nanowire for lithium ion battery [J]. Journal of Power Sources, 2012, 199: 263-269.
[35] LIU Hai-mei, WANG Yong-gang, WANG Kai-xue, HOSONO E, ZHOU Hao-shen. Design and synthesis of a novel nanothorn VO2(B) hollow microsphere and their application in lithium-ion batteries [J]. Journal of Materials Chemistry, 2009, 19(18): 2835-2840.
(Edited by FANG Jing-hua)
中文导读
氢还原钒酸钠纳米线阵列作为锂离子电池的电极
摘要:钒酸盐和氧化钒是潜在的锂离子电池电极材料,具有易于制备和高容量的特点。本文报道了VO2和NaV2O5复合纳米线阵列的电化学锂储存性能。首先,采用水热法制备Na5V12O32纳米线阵列,然后将Na5V12O32纳米线阵列在500 °C的氢气氛中还原反应制备VO2和NaV2O5复合纳米线阵列。对制备样品的晶体结构,化学组成和形态特征进行了表征。结果表明,所得复合纳米线阵列用作锂离子电池的电极,具有高可逆容量和良好的循环稳定性。在电流密度为30 mA/g时经过50次循环后,500 °C下获得的复合物具有高达345.1 mA·h/g的放电比容量。
关键词:钒酸钠;氢还原;纳米线阵列;锂离子电池
Foundation item: Project(51201117) supported by the National Natural Science Foundation of China
Received date: 2018-09-13; Accepted date: 2019-03-08
Corresponding author: FANG Dong, PhD, Associate Professor; Tel: +86-18082733618; E-mail: csufangdong@gmail.com; ORCID: 0000-0003-1292-7770; LU Xue-qin, PhD, Associate Professor; E-mail: luuhee@126.com
Abstract: Vanadates and vanadium oxides are potential lithium-ion electrode materials because of their easy preparation and high capacity properties. This paper reports the electrochemical lithium-storage performance of VO2 and NaV2O5 composite nanowire arrays. Firstly, Na5V12O32 nanowire arrays are fabricated by a hydrothermal method, and then VO2 and NaV2O5 composite nanowire arrays are prepared by a reduction reaction of Na5V12O32 nanowire arrays in hydrogen atmosphere. Crystal structure, chemical composition and morphology of the prepared samples are characterized in detail. The obtained composite is used as an electrode of a lithium-ion battery, which exhibits high reversible capacity and good cycle stability. The composite obtained at 500 °C presents a specific discharge capacity up to 345.1 mA·h/g after 50 cycles at a current density of 30 mA/g.


