
Phase equilibria in Co-rich region of Co-Ti-Ta system
JIANG Min, SAREN Gerile, YANG Su-yu, LI Hong-xiao, HAO Shi-ming
Key Laboratory for Anisotropy and Texture of Materials of Ministry of Education,
Northeastern University, Shenyang 110819, China
Received 30 October 2010; accepted 27 May 2011
Abstract:
The phase equilibria in Co-rich region of Co-Ti-Ta system were studied. The microstructure and XRD analysis together with EDS determination show that L12 type Co3Ti phase and Laves_C36_Co3Ta phase get equilibrium with α-Co phase from 1 000 to 1 200 °C. The Co3Ti phase possesses a solubility of Ta higher than 10%, and the addition of Ta stabilizes the Co3Ti phase. The isothermal sections of the Co-Ti-Ta system in the Co-rich region at 1 000, 1 100 and 1 200 °C were constructed according to the result.
Key words:
L12 type intermetallic compound; phase equilibrium; Co-Ti-Ta; Co-based superalloys;
1 Introduction
The development of superalloys has been driven by the demand to increase the operating temperature of gas turbines in power plants and aircraft engines, thus a higher thermodynamic stability of the γ′ phase is obviously more important. Ta is an important alloying element in both Co-based and Ni-based superalloys, and it has been known that Ta can stabilize the γ′ phase [1-5]. However, both γ′-Ni3Ta and γ′-Co3Ta are not stable, thus, a quantitative thermodynamic description for Ta in stabilizing the γ′ phase is not available.
The L12 type intermetallic compound Co3Ti is the only binary stable γ′ phase in the commercial Co-based superalloys. In many systems, Ta can substitute Ti and exhibits a relatively large solubility region [6-8]. If Ta can substitute Ti in the γ′-Co3Ti phase, then according to thermodynamic analysis [9-11], the thermodynamic stability of the metastable γ′-Co3Ta phase can be estimated.
On the other hand, the information for Co-Ti-Ta system is very limited [12]. Recently, XU et al [13] have studied the phase equilibria in Co-Ti-Ta system at 950 °C and established the phase relationship between α-Co, Co3Ti and Co7Ta2 phases in the Co-rich region. The result is important for the alloy design of the Co-based superalloys, but it is not enough to understand the overall phase relationship in the Co-rich region, especially to establish a critical temperature dependence of the phase stability on the γ′ phase. Therefore, in this work, a systematic experimental study was done to the Co-rich Co-Ti-Ta alloys at 1 000, 1 100 and 1 200 °C to reveal the effect of Ta on the thermodynamic stability of the Co3Ti phase, to determine the Co3Ti phase composition accurately and to establish a reliable phase relationship in the Co-rich region.
The constitute binary systems of Co-Ti and Co-Ta have both been thermodynamically studied before. The Co-Ti system has been thermodynamically assessed by DAVYDOV et al [14]. In the Co-rich region, there is a peritectic reaction of L+α-Co?Co3Ti at 1 181.5 °C, and Co3Ti gets equilibrium with α-Co at low temperature. The binary Co-Ta system has been critically assessed by LIU and CHANG [15] and HARRL et al [16]. In the Co-rich region, the two assessments have no much difference, and both claim that the intermetallic compound Co7Ta2, which gets equilibrium with α-Co, decomposes into α-Co and a Laves_C36 phase Co3Ta at about 950 °C.
2 Experimental
To establish an overall phase relationship in the Co-rich region, alloys with different Co contents in the composition range of Co-Co80Ti20-Co80Ta20 (molar fraction, %, the same as below) were designed. The desired alloys were prepared by high purity metals of Co (99.99%), Ti (99.9%) and Ta (99.9%). The pure metals were melted in an arc furnace under high purity argon atmosphere using a non-consumable tungsten electrode. The buttons were remelted 3 times to improve their homogeneity. The cast alloys were annealed in the quartz tubes evacuated up to 0.3?10-3 Pa at 1 200 °C for 4 h and subsequently quenched into cold water. The alloys for phase equilibrium study at 1 000 and 1 100 °C were further annealed at 1 000 and 1 100 °C for 168 and 72 h, respectively, followed by quenching.
The equilibrated Co-Ti-Ta alloys were first examined using optical microscopy. The microstructures and compositions were then analyzed by scanning electron microscopy (SEM) with the assistance of energy dispersive spectroscopy of X-ray (EDS) on a HITACHI S3400N under an accelerating potential of 20 kV. The phase constituents were determined by X-ray diffraction (XRD) on a Philips PW3040/60 diffractometer using Cu Kα radiation, at a high tension of 40 kV and 40 mA.
3 Results and discussion
3.1 Equilibrium phase constituents
The backscattering electron (BSE) images of the typical alloys annealed at different temperatures are shown in Figs. 1 and 2. Figure 1(a) shows the microstructure of the Co85Ti3Ta12 alloy annealed at 1 200 °C for 4 h. The alloy apparently consists of two phases, the black matrix and the gray precipitated phase. The XRD analysis shows that the matrix is α-Co phase with disordered FCC structure, and the precipitated phase is Laves_C36 phase, denoted as Co3Ta.
The two-phase region of (α-Co+Co3Ta) covers a relatively large composition range in the Co-rich region at temperatures from 1 000 to 1 200 °C. Figures 1(b) and (c) show the microstructures of the Co76Ti5Ta19 and Co85Ti3Ta12 alloys annealed at 1 100 and 1 000 °C, respectively, which are both in the two-phase equilibrium between α-Co and Co3Ta. One difference between them is that the volume fraction of the α-Co phase in the Co76Ti5Ta19 alloy is very small (see Fig. 1(b)), hence the composition of 5% Ti and 19% Ta should be close to the phase boundary of (α-Co+Co3Ta)/Co3Ta. Figures 1(a) and (c) show the microstructure of Co85Ti3Ta12, and in the latter image, the precipitated Co3Ta phase grows apparently during the further heat treatment at 1 000 °C for 168 h after annealing at 1 200 °C for 4 h. XU et al [13] detected the phase equilibrium between α-Co and Co7Ta2 phase at 950 °C. The Co7Ta2 phase decomposes into α-Co and Co3Ta just at about 950 °C according to the binary Co-Ta phase diagram [15], thus a two-phase equilibrium between α-Co and Co3Ta is obtained at 1 000 °C in the Co-Ta side.
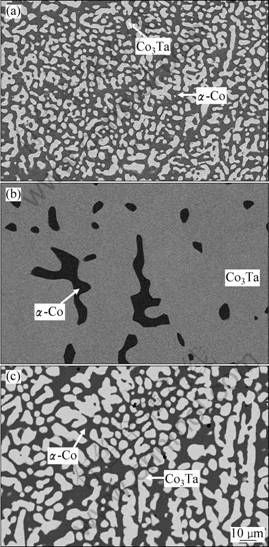
Fig. 1 SEM images of Co85Ti3Ta12 alloy annealed at 1 200 °C for 4 h (a), Co76Ti5Ta19 alloy annealed at 1 100 °C for 72 h after heat treatment at 1 200 °C for 4 h (b) and Co85Ti3Ta12 alloy annealed at 1 000 °C for 168 h after heat treatment at 1 200 °C for 4 h (c)
With the increase of Ti, the Co-Ti-Ta alloys leave from the (α-Co+Co3Ta) two-phase region. Figure 2 shows the microstructures of the Co76Ti12Ta12 and Co73Ti16Ta11 alloys annealed at 1 100 °C for 72 h after heat treatment at 1 200 °C for 4 h. The first alloy enters into a three-phase region, consisting of dark α-Co phase, grey Co3Ta phase and another dark grey Co3Ti phase, based on the XRD analysis results. The latter alloy with more addition of Ti has no α-Co phase, and the alloy is located in a two-phase region of (Co3Ta+Co3Ti). The phase constitution of the alloys annealed at 1 000 °C is almost the same as that annealed at 1 100 °C, however, those of alloys with higher Ti content annealed at 1 200 °C are different. At about 1 180 °C, a peritectic reaction L+α-Co?Co3Ti occurs in the binary Co-Ti system [3], thus the Co-Ti-Ta alloys with higher Ti content have liquid at 1 200 °C first at the phase boundaries.
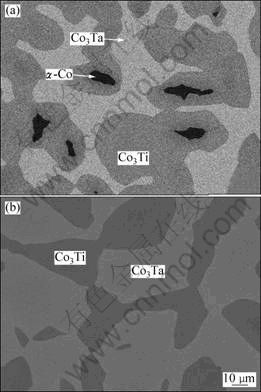
Fig. 2 SEM images of alloys annealed at 1 100 °C for 72 h after heat treatment at 1 200 °C for 4 h: (a) Co76Ti12Ta12 alloy; (b) Co73Ti16Ta11 alloy
3.2 Equilibrium phase compositions
Based on the microstructure observation and XRD analysis above, the phase constituents of the treated alloys can be decided. The phase compositions are further determined by SEM-EDS. It should be mentioned that all these results show that the treated alloys have come to a good equilibrium, so the measured phase compositions can be regarded as the equilibrium phase compositions as listed in Table 1.
3.3 Isothermal sections
According to the microstructure analysis performed to the equilibrium Co-Ti-Ta alloys, the isothermal sections in the Co-rich region of the Co-Ti-Ta system at 1 000, 1 100 and 1 200 °C can be established, as shown in Fig. 3. The tie lines and tie triangles corresponding to the two-phase and three-phase equilibrium compositions are from the data listed in Table 1. It can be seen that the solubilities of Ti and Ta in the α-Co phase have been well determined. The Co3Ta phase is detected to have a very high solubility of Ti at these temperatures. In fact, the Co3Ta phase is reported to form a continuous ternary solid solution extending from the Laves_C36_Co3Ta to the Laves_C36_Co2Ti phase [13]. The Co3Ti phase also has a certain solubility of Ta. At 1 000 °C, it can dissolve more than 10% Ta, while at 950 °C, the solubility of Ta is reported to be slightly higher than 10%, which is in a good agreement with this work. It is obvious that Ta can stabilize the γ′-Co3Ti phase, and the composition range of the Co3Ti phase presents like a narrow strip pointing to the Co75Ta25 composition, with Co content remaining at about 75%. Then it is deduced that Ta mainly substitutes Ti in the γ′-Co3Ti phase.
Table 1 Equilibrium phase constituents and compositions in Co-rich Co-Ti-Ta system
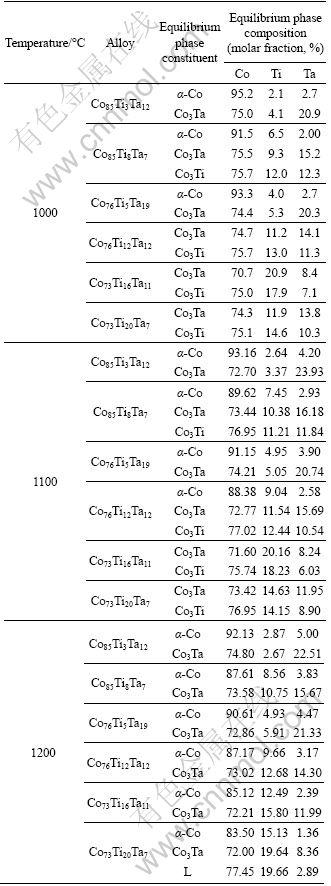
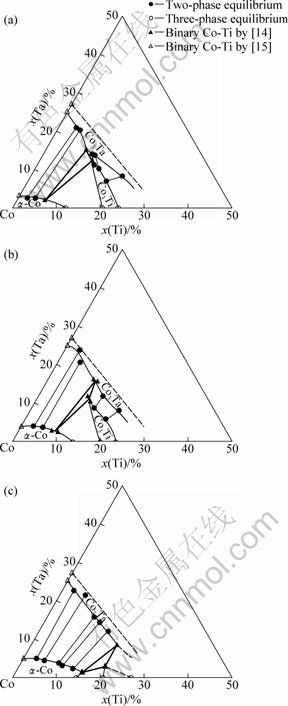
Fig. 3 Isothermal sections in Co-rich region of Co-Ti-Ta system: (a) 1 000 °C; (b) 1 100 °C; (c) 1 200 °C
The fact that Ta can substitute Ti in γ′-Co3Ti phase more than 10% at 1000 °C means there should exist a metastable γ′-Co3Ta phase in the Co-Ta binary system. From the view point of thermodynamics, due to the metastable γ′-Co3Ta phase as well as the entropy increased from the mixing of Ti and Ta in the same atomic lattice of Ti, Ta exhibits a stabilizing effect on the γ′-Co3Ti phase. With the reliable phase relationship in the Co-Ti-Ta system discussed above, a thermodynamic description of the metastable γ′-Co3Ta phase can be estimated, which will be done in further work.
4 Conclusions
1) In the Co-rich region of the Co-Ti-Ta system, the L12 type Co3Ti phase and the Laves_C36_Co3Ta phase get equilibrium with the α-Co phase from 1 000 to 1 200 °C.
2) The Co3Ti phase is detected to possess a solubility of Ta higher than 10%. The addition of Ta makes the Co3Ti phase more stable, and Ta mainly substitutes Ti in the L12 type Co3Ti phase.
3) Isothermal sections in the Co-rich region of the Co-Ti-Ta system at 1 000, 1 100 and 1 200 °C are constructed based on the measurements in this work. The determined solid solubilities of the α-Co phase, L12 type Co3Ti phase and the Laves_C36_Co3Ta phase are in good agreement with the previous determination.
References
[1] SATO J, OMORI T, OIKAWA K, OHNUMA I, KAINUMA R, ISHIDA K. Cobalt-based high-temperature alloys [J]. Science, 2006, 312(5770): 90-91.
[2] JIA C C, ISHIDA K, NISHIZAWA T. Partition of alloying elements between γ(A1), γ′(L12) and β(B2) phases in Ni-Al based systems [J]. Metallurgical and Materials Transaction A, 1994, 25(3): 473-485.
[3] SUZUKI A, POLLOCK T M. High-temperature strength and deformation of γ/γ′ two-phase Co-Al-W-base alloys [J]. Acta Materialia, 2008, 56(6): 1288-1297.
[4] OOSHIMA M, TANAKA K, OKAMOTO N, KISHIDA K, INUI H. Effects of quaternary alloying elements on the γ′ solvus temperature of Co-Al-W based alloys with fcc/L12 two-phase microstructures [J]. Journal of Alloys and Compounds, 2010, 508(1): 71-78.
[5] CHEN M, WANG C Y. First-principles investigation of the site preference and alloying effect of Mo, Ta and platinum group metals in gamma γCo3(Al, W) [J]. Scripta Materialia, 2009, 60(8): 659-662.
[6] LIU Y J, ZHANG L J, DU Y, WANG J, LIANG D. Study of atomic mobilities and diffusion characteristics in bcc Ti-Ta and Ta-W alloys [J]. CALPHAD, 2010, 34(3): 310-316.
[7] WITUSIEWICZ V T, BONDAR A A, HECHT U, VOBLIKOV V M, FOMICHOV O S, PETYUKH V M, REXA S. Experimental study and thermodynamic modelling of the ternary Al-Ta-Ti system [J]. Intermetallics, 2011, 19(3): 234-259.
[8] BUENCONSEJO P J S, KIM H Y, MIYAZAKI S. Effect of ternary alloying elements on the shape memory behavior of Ti-Ta alloys [J]. Acta Materialia, 2009: 57(8): 2509-2515.
[9] HAO Shi-ming, JIANG Min, LI Hong-xiao. Materials Thermodynamics [M]. Beijing: Chemical Industry Press, 2010: 276-284. (in Chinese)
[10] MATHON M, CONNETABLE D, SUNDMAN B, LACAZE J, Calphad-type assessment of the Fe-Nb-Ni ternary system [J]. CALPHAD, 2009, 33(1): 136-161.
[11] ABE T, SUNDMAN B. A description of the effect of short range ordering in the compound energy formalism [J]. CALPHAD, 2003, 27(4): 403-408.
[12] KUO K. Ternary laves and sigma-phases of transition metals [J]. Acta Metallurgica, 1953, 1: 720-724.
[13] XU Hong-hui, XIONG Xiang, DU Yong, WANG Pei-sheng, HU Biao, HE Yue-hui. Phase equilibria of the Co-Ta-Ti system at 950 °C [J]. Journal of Alloys and Compounds, 2009, 485(1-2): 249-254.
[14] DAVYDOV A V, KATTNER U R, JOSELL D, BLENDELL J E, WATERSTRAT R M, SHAPIRO A J, BOETTINGER W J. Determination of the CoTi congruent melting point and thermodynamic reassessment of the Co-Ti system [J]. Metallurgical and Materials Transaction A, 2001, 32(9): 2175-2186.
[15] LIU Z K, CHANG Y A. Thermodynamic assessment of the Co-Ta system [J]. CALPHAD, 1999, 23(3-4): 339-356.
[16] HARRI KUMAR K C, VAN ROMPAEY T, WOLLANTS P. Thermodynamic calculation of the phase diagram of the Co-Nb-Ta system [J]. Zeitschrift fur Metallkunde, 2002, 93(11): 1146-1153.
Co-Ti-Ta三元系富Co区的相平衡
蒋 敏,萨仁格日乐,杨舒宇,李洪晓,郝士明
东北大学 材料各向异性与织构教育部重点实验室,沈阳 110819
摘 要:研究Co-Ti-Ta三元系富Co区的相平衡。显微组织和XRD分析以及EDS检测结果表明,在1 000~1 200 °C温度范围内,L12结构Co3Ti相和Laves_36_Co3Ta相与α-Co构成相平衡。Co3Ti相中Ta的固溶度超过10%,Ta的加入使Co3Ti相更稳定。根据实验结果构建Co-Ti-Ta三元系富Co区在1 000、1 100和1 200 °C等温截面图。
关键词:L12型金属间化合物;相平衡;Co-Ti-Ta;钴基高温合金
(Edited by FANG Jing-hua)
Foundation item: Project (50771027) supported by the National Natural Science Foundation of China
Corresponding author: JIANG Min; Tel: +86-24-83681676; E-mail: jiangm@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61025-X
Abstract: The phase equilibria in Co-rich region of Co-Ti-Ta system were studied. The microstructure and XRD analysis together with EDS determination show that L12 type Co3Ti phase and Laves_C36_Co3Ta phase get equilibrium with α-Co phase from 1 000 to 1 200 °C. The Co3Ti phase possesses a solubility of Ta higher than 10%, and the addition of Ta stabilizes the Co3Ti phase. The isothermal sections of the Co-Ti-Ta system in the Co-rich region at 1 000, 1 100 and 1 200 °C were constructed according to the result.


