
Study of spent battery material leaching process
LI Jin-hui(李金辉)1, 2, LI Xin-hai(李新海)1, ZHANG Yun-he(张云河)1, HU Qi-yang(胡启阳)1,
WANG Zhi-xing(王志兴)1, ZHOU You-yuan(周友元)1, FU Fang-ming(符芳铭)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Materials and Chemical Engineering, Jiangxi University of Science and Technology,
Ganzhou 341000, China
Received 3 July 2008; accepted 5 January 2009
Abstract:
The recovery of Ni, Co and Mn from spent battery material is very important to environment protection, utilization of resources and cost reduction of the material. The dissolution rates of Ni, Co and Mn with hydrochloric acid as leachant are all over 95% under the optimal conditions of initial hydrochloric acid of 6 mol/L, particle size of 120 μm for the exhausted scraps, molar ratio of H2O2 to MeS of 2, leaching temperature about 60 ℃, ratio of liquid to solid of 8, and leaching time of 2 h. The NixCoyMnz precursor for cathode material prepared from the purified leaching solution, can meet the demand of precursor by pure chemicals. The process is economic and feasible for base metals from spent battery material.
Key words:
spent battery materials; nickel; cobalt; manganese; recovery; leaching;
1 Introduction
Nickel and cobalt are very important in many fields, and the world’s demand for them is progressively increasing. Due to the primary resources being depleted and the constraints by the laws and legislation related to the environment protection in the world, it becomes more and more important to recover these metals as the secondary resources. HU et al[1] extracted these metals from waste catalyst, GBOR et al[2] recovered Ni and Co from waste nickel smelt slag, and RABAH et al[3] recovered Ni, Co and some salts from spent Ni-MH batteries. Many methods have been applied for treatment of these spent materials and subsequent separation of impurities from leaching solution, such as leaching with different acids, chlorination, pyro- and hydro- metallurgical processes for extraction[4-9], and solvent extraction[10-11], ion exchange[12-14], sulfuration precipitation[15].
As cathode material for lithium ion battery, NixCoyMnz is widely applied in the battery industry[16]. Traditionally, the cathode material is prepared by using pure chemical reagents. To reduce the cost of the materials, it is essential to employ the secondary resources. LIU et al[17] prepared satisfactory LiCoO2 from battery castoff. QIN et al[18] obtained lead sulfate powders from galena concentrates directly. The aim of this work is to recover nickel, cobalt, manganese and other metals from spent battery material by leaching treatment. The effects of various conditions, such as the ratio of liquid to solid(L/S), leaching temperature, molar ratio of H2O2 to MeS, acidity, oxidant concentration and particle size are investigated, and the precursor of NixCoyMnz cathode material is prepared directly from leaching solution.
2 Experimental
2.1 Materials
The composition of spent battery materials from a battery plant in Hunan Province, China, is shown in Table 1. The materials were mainly made of electrode castoffs including oxides of cobalt, nickel and manganese as well as their mixture with some alloy scraps used in production of battery; the acid insoluble components consisted of silica and refractory materials from electrode supplies in sintering. Fig.1 shows that XRD pattern of the spent materials, where the main components are cobalt oxide, nickel oxide, manganese sulfide, ferric and ferrous oxide, copper oxide and copper iron sulfide. The samples were ground and sieved into four groups: 200, 150, 120 and 80 μm. Chemical grade hydrochloric acid was used in this study.
Table 1 Chemical composition of spent battery materials (mass fraction,%)


Fig.1 XRD pattern of spent battery material
2.2 Principle
Some reactions occurring in the leaching process are as follows:
Fe3O4+8H+=2Fe3++Fe2++4H2O (1)
CoO+2H+=Co2++H2O (2)
NiO+2H+=Ni2++H2O (3)
MnS2+4H2O2=Mn2++SO42-+4H2O+S↓ (4)
2Cu5FeS4+31H2O2+14HCl=
6CuSO4+4CuCl2+ 2FeCl3+38H2O+2S↓ (5)
2.3 Procedure
The effects of leaching parameters such as initial HCl concentration, L/S ratio, temperature, molar ratio of H2O2 to MeS, particle size and leaching time on dissolution rate of the main metals in the samples were investigated. The prepared leaching solution with the desired hydrochloric acid concentration was placed into a 1 L round-bottom flask with 3 holes. Once the desired temperature was achieved, the dried sample was decanted to the flask from the feed opening and stirred at a constant speed by a Te?on coated magnet. The reaction temperature was controlled by an isothermal water bath with a digital thermometer (within ±1 ℃). The reaction mixture was agitated at a fixed rate of 250 r/min. The effect of leaching time on metallic extraction was calculated by Eq.(6). At the end of leaching, the leached residue was ?ltered and washed with distilled water using a Büchner funnel. The leaching rates of metals (Ni, Co, etc) were calculated by detecting the contents of metals in the filtrate.
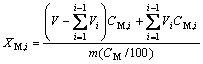 (6)
(6)
where XM,i is the dissolution rate of M (Ni, Co, etc) in the sample i withdrawn each time, V the initial volume of the solution, Vi the volume of the sample i, CM,i the content of M in sample i, m the initial mass of waste (on dried basis) added in the reactor and CM the content of M in waste.
2.4 Analysis
The contents of Co, Cu, Ni, Fe and Mn in samples and solution were analyzed by a chemical method and atomic absorption spectrophotometry (Ruili-wfx120). X-ray diffractometry (Rint-2000, Rigaku) was used to identify the components of the spent battery and leaching residue.
3 Results and discussion
3.1 Effect of initial HCl concentration
The effect of HCl concentration on the leaching of valuable metals is shown in Fig.2. It can be seen that the leaching rates increase gradually with the increase of initial HCl concentration. The increase in the amount of acid can increase the dissolution rate based on kinetics [19]. The maximum dissolution rate can reach 96.5%, 95.5%, 98.5%, 96.0% and 96.3% for nickel, cobalt, copper, manganese and iron, respectively. The maximum rates of these metals except iron were increased little when the acid concentration was beyond 6 mol/L. This is probably because the determination of iron is interfered by its complex with chloride ion. The optimum initial HCl concentration is chosen to be 6 mol/L.

Fig.2 Effect of initial HCl concentration on dissolution rates of Ni, Co, Mn, Cu and Fe under conditions of L/S ratio of 8/1, molar ratio of H2O2 to MeS of 2, particle size of 120 μm at 60 ℃ for 120 min
3.2 Effect of L/S ratio
Fig.3 shows that the dissolution rates increase obviously with the increase of L/S ratio but do not when the ratio is beyond 8:1. The increase in L/S ratio could decrease the concentration of metal ion in leaching solution, strengthen mass transfer and accelerate the leaching rate[19]. But too high L/S ratio would lead to the increase of leaching solution volume, which is not favorable to subsequent separation. Therefore, the L/S ratio of 8:1 is suitable.
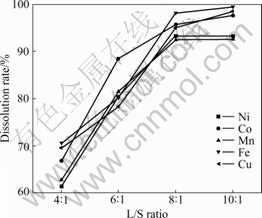
Fig.3 Effect of L/S ratio on dissolution rates of Ni, Co, Mn, Cu and Fe under conditions of initial HCl concentration of 6 mol/L, molar ratio of H2O2 to MeS of 2, particle size of 120 μm at 60 ℃ for 120 min
3.3 Effect of H2O2 content
It can be observed in Fig.4 that the content of oxidant H2O2 can affect the dissolution rate greatly. The dissolution rates of all metals increase with the increase of H2O2. In this leaching process, sulfide ion generates and reacts with metal ion and hydrogen ion to form insoluble sulfide and to release gas. To improve the dissolution rate, one way is to increase the acid concentration, which can be proved in Fig.2, the other is to add oxidant to make sulfide ion to form sulfate[20]. The dissolution rates of Fe, Mn and Cu increase more rapidly than those of Ni and Co as the oxidant content increases. This is because part of Fe, Mn and Cu exist in sulfide forms whilst Ni and Co exist in oxide form in waste as shown in Fig.1. When the molar ratio of H2O2 to MeS is more than 2, the maximum dissolution rate can be obtained.

Fig.4 Effect of molar ratio of H2O2 to MeS on dissolution rates of Ni, Co, Mn, Cu and Fe for samples with particle size of 120 μm under conditions of initial HCl concentration of 6 mol/L, L/S ratio of 8/1 at 60 ℃ for 120 min
3.4 Effect of temperature
The effect of temperature on dissolution rate is shown in Fig.5. It is clearly observed that the dissolution rate increases with temperature increasing from 20 to 60 ℃. The dissolution rate of Ni increases from 90% to 97%, Co from 86% to 95%, Mn from 82% to 96%, respectively. However, the dissolution rates of Ni and Co are not greatly affected by temperature. It is well known that Ni and Co mainly exist in samples as oxide form which can be easily dissolved by acid. But, parts of Fe, Cu and Mn as sulfides can be extracted in the presence of oxygen or some other oxidant[9]. Hence, the reactive activity of oxidants is intensified greatly when temperature increases. Although the dissolution rate can be improved with increase in temperature, the dissolution rates of Fe, Cu and Mn begin to descend at 90 ℃. Besides, at high temperature the operation cost increases and hydrochloric acid poignantly evaporates, which results in the operation conditions worse. So, 60 ℃ is chosen as an optimum temperature.
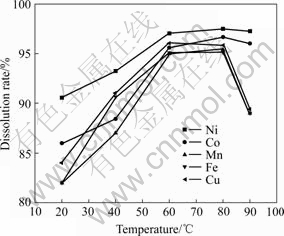
Fig.5 Effect of temperature on dissolution rates of Ni, Co, Mn, Cu and Fe under conditions of initial HCl concentration of 6 mol/L, particle size of 120 μm, molar ratio of H2O2 to MeS of 2 and L/S ratio of 8/1 for 120 min
3.5 Effect of particle size
The effect of particle size on the dissolution rates is presented in Fig.6. It is indicated that the dissolution rates of these metals are fairly similar, i.e. they increase with the decrease of particle size and reach about 95%. The decrease of particle size can increase the surface area of mineral, which increases the contact chance between solid and solution, leading to the increase of dissolution rate in given conditions. To acquire satisfactory leaching rate for the tested metals, it is necessary to keep particle size below 120 μm.
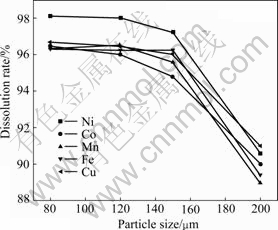
Fig.6 Effect of particle size on dissolution rates of Ni, Co, Mn, Cu and Fe under conditions of molar ratio of H2O2 to MeS of 2, initial HCl concentration of 6 mol/L, L/S ratio of 8/1 at 60 ℃ for 120 min
3.6 Effect of leaching time
The effect of leaching time on the dissolution rates is shown in Fig.7. It can be seen that the dissolution rates of Ni and Co can achieve a high value in 40 min and the maximum value of Ni is presented after leaching for 60 min. Because Ni and Co exist in oxide forms (Fig.8), the interaction of acid with relatively high concentration to oxides of Ni and Co is fast, and the leaching rates of them reach the maximum in relatively short leaching time[21]. For Fe and Co exist in oxide and sulfide forms (see Fig.8), the metal oxides can react with HCl, while the sulfides are oxidized by H2O2 in the initial period. As shown in Fig.7, with the leaching proceeding, the oxidized sulfides dissolve gradually, and the leaching rates of Fe and Cu increase continuously in the whole leaching process. MnS2 reacts with H2O2 in acid solution, the Mn2O3 solid generates and dissolves gradually. It can be observed from the appearance and disappearance of X-ray diffraction peaks for the Mn2O3 at 2q angle of 34.1? in Fig.8. Since both precipitation and dissolution occur simultaneously, a mediate plateau presents in the leaching rates curve of Mn, as shown in Fig.7.
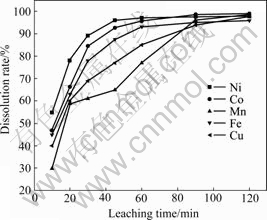
Fig.7 Effect of leaching time on dissolution rates of Ni, Co, Mn, Cu and Fe under conditions of molar ratio of H2O2 to MeS of 2, initial HCl concentration of 6 mol/L, L/S ratio of 8/1, particle size of 120 μm at 60 ℃
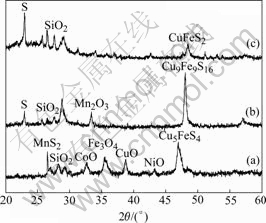
Fig.8 XRD patterns of material and leaching residue: (a) Material; (b) Leaching residue after 1 h; (c) Leaching residue after 2 h
4 Purification
There are large quantities of valuable metals, such as Ni, Co, Mn, Cu and Fe, as well as impurities in leaching solution. Cu was first removed through replacement by iron powder, then iron was removed by precipitation as goethite. After purification, the solution contains Ni 18.6 g/L, Co 18.1 g/L and Mn 4 g/L. According to NixCoyMnz ratio, the given amounts of chlorides of nickel, manganese and cobalt were added into the solution to prepare the precursor of the cathode material directly through co-precipitation with ammonium bicarbonate. The precursor can meet the demand of precursor by pure chemical.
5 Conclusions
To prepare battery material from secondary resources, the optimum leaching conditions were obtained with spent battery material as resources. The optimum leaching conditions for the spent battery material are: temperature 60 ℃, molar ratio of H2O2 to MeS 2, initial HCl concentration 6 mol/L, L/S ratio 8/1, particle size less than 120 μm and leaching time 120 min. The leaching solution after purification and separation of impurities can be used to prepare the precursor of NixCoyMnz cathode material directly. The precursor can be up to standard of precursor made by pure chemical, and is competitive of cost with the chemicals prepared from primary resources.
References
[1] HU Jian-feng, ZHU Yun, HU Han. Comprehensive recovery of vanadium, molybdenum and nickel from dead catalyst [J]. Chinese Journal of Rare Metals, 2006, 30(5): 711-714. (in Chinese)
[2] GBOR P K, AHMED I B, JIA C Q. Behaviour of Co and Ni during aqueous sulphur dioxide leaching of nickel smelter slag [J]. Hydrometallurgy, 2000, 57(1): 13-22.
[3] RABAH M A, FARGHALY F E, ABD-El MOTALEB M A. Recovery of nickel, cobalt and some salts from spent Ni-MH batteries [J]. Waste Management, 2008, 28(7): 1159-1167.
[4] FANG Cheng-kai, TAN Pei-jun. Separation and recovery of cobalt and nickel from electro-solution of Co-Ni waste [J]. Hydrometallurgy of China, 2003, 22(4): 169-182. (in Chinese)
[5] XIE Fu-biao. Practice on comprehensive recovery of valuable metals from cobalt-bearing wastes [J]. Mining & Metallurgy, 2001, 10(3): 61-64. (in Chinese)
[6] LIU Yan, WANG Hong, JIANG Gui-quan, ZHANG Guang-li. Acid leaching of a nickelferrous spent material and the effect of circular countercurrent leaching [J]. Mining & Metallurgy, 2006,26(5): 44-46. (in Chinese)
[7] XIA Yu, HUANG Mei-song, YANG Xiao-zhong, MAO Yong-jun, LI Xian-bai. The study of preparation of electron-level nickel sulphate using positive electrodes of spent Ni-MH batteries [J]. Mining & Metallurgy, 2005, 25(4): 46-49, 53. (in Chinese)
[8] PENG Bing, GAO Hui-mei, CHAI Li-yuan, SHU Yu-de. Leaching and recycling of zinc from liquid waste sediments [J]. Trans Nonferrous Met Soc China, 2008, 18(5): 1269-1274.
[9] YUNJIAO L, ILYA P, VLADIMIROS G, PAPANGELAKIS. Cleaning of waste smelter slags and recovery of valuable metals by pressure oxidative leaching [J]. Journal of Hazardous Materials, 2008, 152(2): 607-615.
[10] FLETT D S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants [J]. Journal of Organometallic Chemistry, 2005, 690(10): 2426-2438.
[11] SHEN Yong-feng, XUE Wen-ying, NIU Wen-yong. Recovery of Co(Ⅱ) and Ni(Ⅱ) from hydrochloric acid solution of alloy scrap [J]. Trans Nonferrous Met Soc China, 2008, 18(5): 1262-1268.
[12] MENDES F D, MARTINS A H. Selective nickel and cobalt uptake from pressure sulfuric acid leach solutions using column resin sorption [J]. Int J Miner Process, 2005, 77(1): 53-63.
[13] GIANNOPOULOU I, PANIAS D. Differential precipitation of copper and nickel from acidic polymetallic aqueous solutions [J]. Hydrometallurgy, 2008, 90(2/4): 137-146.
[14] SEGGIANI M, VITILO S, ANTONE S D. Recovery of nickel from orimulsion fly ash by iminodiacetic acid chelating resin [J]. Hydrometallurgy, 2006, 81(1): 9-14.
[15] HENRY H T, RICHARD S, JOHN B. Advances in biotreatment of acid mine drainage and biorecovery of metals (1): Metal precipitation for recovery and recycle [J]. Biodegradation, 2003, 14(3): 423-426.
[16] ZHANG Bao, ZHANG Ming, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, PENG Wen-jie. Synthesis and electrochemical properties of LiNi0.45Co0.10Mn0.45O2 cathode material for lithium ion batteries [J]. Journal of Central South University: Science and Technology, 2008, 39(1): 75-79. (in Chinese)
[17] LIU Yun-jian, HU Qi-yang, LI Xin-hai, GUO Hua-jun, WANG Zhi-xing. Synthesis and electrochemical behavior of LiCoO2 recycled from incisors bound of Li-ion batteries [J]. Trans Nonferrous Met Soc China, 2007, 17(S1): 902-906.
[18] QIN Wen-qing, LIU Hui, SUN Wei. Method of direct preparation of lead sulfate powders from galena concentrates [J]. Journal of Central South University: Science and Technology, 2005, 36(3): 407-411. (in Chinese)
[19] RUBISOV D H, KROWINKEL J M, PAPANGELAKIS V G. Sulphuric acid leaching of laterites-universal kinetics of nickel dissolution for limonites and limonitic/saprolitic blends [J]. Hydrometallurgy, 2000, 58(1): 1-11.
[20] HERREROS O, QUIROZ R, MANZANO E, BOU C, VINALS J. Copper extraction from reverberatory and ?ash furnace slags by chlorine leaching [J]. Hydrometallurgy, 1998, 49(1/2): 87-101.
[21] GBOR P K, MOKRI V, JIA C Q. Characterization of smelter slags [J]. J Environ Sci Health A, 2000, 35(2): 147-167.
Foundation item: Project(2007CB613607) supported by the National Basic Research Program of China; Project(50864004) supported by the National Natural Science Foundation of China
Corresponding author: LI Xin-hai; Tel: +86-731-8836633; E-mail: bigworld_li@163.com
DOI: 10.1016/S1003-6326(08)60345-3
(Edited by YUAN Sai-qian)


