
Phase selection in highly undercooled Fe-B eutectic alloy melts
YANG Chang-lin(杨长林), YANG Gen-cang(杨根仓), LU Yi-ping(卢一平),
CHEN Yu-zeng(陈豫增), ZHOU Yao-he(周尧和)
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 23 March 2005; accepted 4 November 2005
Abstract:
The high undercooling technique by molten glass slag purification and cyclical superheating in Ar atmosphere was applied to bulk Fe-B alloy melts. A hypercooling was achieved which suppressed the formation of stable phase and consequently promoted the nucleation of metastable phase. Fe-17%B and Fe-20%B alloys were investigated, respectively. TEM and X-ray powder diffraction analyses verify the formation of metastable phase in the highly undercooled Fe-B alloy melts. Besides, the critical nucleation work of Fe2B and Fe3B phases was calculated to predict phase selection in the undercooled melts. The results show that the metastable phase formation is a function of the undercooling achieved prior to nucleation. And the amount of undercooling is an important factor in determining microstructural development by controlling phase selection in the undercooled melts.
Key words:
Fe-B eutectic alloy; metastable phase; phase selection; hypercooling;
1 Introduction
Phase selection processes under rapid solidification conditions do not solely depend on composition but on process parameters such as melt undercooling prior to solidification and cooling velocity[1]. However the environment cooling conditions and volume of samples have limited the increasing of cooling velocity in bulk melts, which makes it more difficult to clarify phase selection mechanism in the rapidly solidified bulk melts. On the contrary, these limitations have almost no influence on the development of undercooling. Then it is possible to control phase selection of alloy melts with high undercooling method in the melt. At present, undercooling technique has been successfully applied to control the nucleation of alloy melt. According to metastable phase diagrams when melt undercooling exceeds a certain critical undercooling, metastable phases will form. It has been confirmed by the experiments in Fe-based, Ni-V, Nd-Fe-B[2], Nb-Al[3], Ni-Si[4] and Ni-Nb[5] alloys with a competitive nucleation of metastable bcc, stable fcc and some intermetallic phases, respectively. But metastable phases obtained in undercooled melt often decompose and transform into stable phases during the slow post-solidification. These questions hinder metastable phase characteristic investigation and the correlation description between melt undercooling and phase selection. Fortunately, hypercooling solved the problems of metastable phase decomposing and transformation. With this method, metastable phase can be not only obtained directly from alloy melt but also kept in the solidified samples, which provides direct experimental evidence for phase selection. It is well known that in general undercooling experiments crystalline phase is remelted due to recalescence during nucleating, which decreases cooling velocity and provides thermo- dynamic condition for phase decomposing and transformation. Now, we assume that the undercooling of melt is sufficiently high, the equilibrium phases are suppressed and the metastable phases are developed. On the other hand, remelting of melt is avoided, thus, the metastable phases formed in undercooled melt will grow preferentially without remelting. This undercooling is defined as the hypercooling. It can be realized by the special molten glass slag purification incorporated with cyclical superheating technique.
In the present paper, Fe-B eutectic alloy was selected due to known metastable phase(Fe3B) reported in this alloy system by conventional rapid solidification processing and other techniques[6-8]. Relationship between the phase selection and undercooling in undercooled alloy melts will be investigated systematically by hypercooling method. The metastable phase (Fe3B) formed in the hypercooled Fe-B eutectic alloy melt will be analyzed by transmission electron microscopy(TEM) and X-ray powder diffractometry (XRD). The microstructure evolution of metastable phase (Fe3B) under hypercooling condition will also be studied. Moreover, to understand the completive nucleation between the metastable phase and stable phase, nucleation work calculations were performed on the Fe -B eutectic alloy.
2 Experimental
5 g samples were prepared from constituent of purity better than 99.8% Fe and 99.5% boron in high- frequency induction furnace in argon atmosphere. The different undercooling conditions were established by the application of special molten glass slag purifica- tion incorporated with cyclical superheating technique. The temperatures of melt were measured contactlessly by an infrared pyrometer during the entire experiment cycle in order to record the thermal behavior of the sample. The melting temperature and undercooling in the cooling curve could be obtained from the compari- son of the absolute temperature.
3 Results and discussion
3.1 Microstructure and phase analysis of Fe-17%B (mole fraction) and Fe-20%B samples
From the equilibrium phase diagram[9] we expect a co-operative eutectic growth of the α-Fe and Fe2B phases for the Fe83B17 alloy. However, in present hypercooling experiments, when the undercooling of melt ΔT<386 K it is coincident with equilibrium phase composition. Fe2B andα-Fe phases occur in the microstructure without other phases by the X-ray diffraction analyses (Fig.1). When ΔT≥386 K, the X-ray diffraction patterns display no trace of any stable phase (Fe2B) in addition to the α-Fe and metastable phase Fe3B. Even when ΔT=485 K, the metastable phase Fe3B was detected in the hypereutectic alloy (Fig.1, Fe-20%B). Additionally, alloy compositions have also been analyzed, which are consistent with original compositions. This implies that hypercooling does not change alloy compositions. And alloy cooling is completed under room temperature condition. This excludes influence of cooling velocity, too. So we may draw a conclusion that hypercooling make alloy melts far from equilibrium and strongly influence the micro- structure of the solidified melts, and there is a hyper- cooling range for metastable phase formation.
The hypercooling of alloy melts can be calculated by equation as where ΔH is alloy fusion enthalpy. cp is heat capacity of liquid. The hypercooling of Fe-B eutectic alloy is about 300 K by Eqn.(1)[6]. That is to say the melt was hypercooling state when ΔT is larger than 300 K. But in rapid solidification processing, phase selection from deeply undercooled melts is determined by the kinetics of nucleation and growth for either the equilibrium solid or one of several possible metastable phases. Here, 300 K is not the critical hypercooling to form the metastable phase due to the insufficient kinetic condition. From the thermodynamic point view, undercooling is a necessary precondition for the solidification of metastable phases[1], which is revealed in the metastable phase diagram that metastable eutectic temperature is below stable eutectic temperature[6]. However, hypercooling is a necessary kinetic condition for controlling metastable phase transformation. In present work, only when the hypercooling exceeds 386 K, metastable phase Fe3B can obtain sufficient driving force for crystallization and growth. That is to say hypercooling prompts greatly solidification velocity, and then Fe3B has no sufficient time to decompose and is preserved to room temperature. In addition, Eqn.(1) is based on equilibrium phase diagram, which makes hypercooling value small in comparison with experimental result.
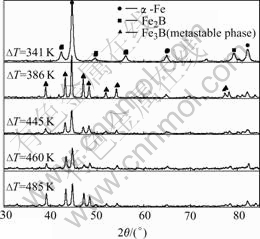
Fig.1 XRD patterns of Fe83B17 and Fe80B20 alloy solidified under undercoolings of ΔT=341 K, ΔT=386 K, ΔT=445 K, ΔT=460 K(Fe83B17) and ΔT=485 K (Fe80B20)
ΔThyper=ΔH/cp (1)
Fig.2 shows the TEM diffraction patterns of stable phase Fe2B, the metastable crystalline phase Fe3B and α-Fe phase. By the TEM diffraction patterns and XRD analysis, it has been confirmed that the metastable phase Fe3B and stable phase Fe2B are approximately tetragonal structure, but their atomic arrangement and lattice constant are quite different (![]() =0.868 99 nm,
=0.868 99 nm, ![]() =0.431 28 nm,
=0.431 28 nm, ![]() = 0.511 03 nm,
= 0.511 03 nm, ![]() =0.424 94 nm[10, 11]). This deter- mines Fe3B and Fe2B have some similar nucleation and crystalline characteristics. In such cases, both competitive nucleation and competitive growth of metastable phase Fe3B and table phase Fe2B will be involved, which will be investigated in the further works.
=0.424 94 nm[10, 11]). This deter- mines Fe3B and Fe2B have some similar nucleation and crystalline characteristics. In such cases, both competitive nucleation and competitive growth of metastable phase Fe3B and table phase Fe2B will be involved, which will be investigated in the further works.
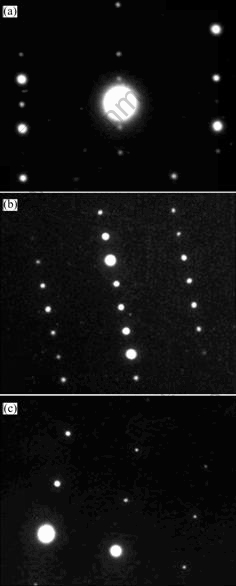
Fig.2 TEM diffraction patterns of as-solidified Fe83B17: (a) Stable phase Fe2B phase diffraction pattern; (b) Metastable phase Fe3B diffraction pattern; (c) α-Fe diffraction pattern
By TEM, it has also been identified that the gray massive phases are Fe2B in Fig.3(a) and Fe3B in Figs.3(b), (c), (d), (e), and matrix phase is α-Fe phase. Additionally, the hypercooling extends eutectic intergrowth zone, so hypereutectic alloy can also achieve eutectic structure. In Fig.3(e) the hypereutectic structure is replaced by the eutectic structure due to the primary phase is suppressed.
It can be seen from Fig.3 that an anomalous eutectic microstructure is visible with increasing undercooling (ΔT≥341 K). Though formation mechanism of anomalous eutectic microstructure does not have a clear explanation, it can be understood that primary phase forms dendritic framework and another phase nucleates in interdendritic liquid. Especially under hypercooling condition, the hypercooling supplies sufficient driving forces for Fe3B nucleation and growth. As a leading phase, Fe3B phase grows rapidly to form the solid-liquid interface, resulting in the enrichment of the second phase on the liquid phase boundary layer. Thus the metastable phase Fe3B forms the interpenetrating frameworks, while α-Fe phase nucleates and grows among these frameworks. In one word, Fe3B phase builds an eutectic framework, the second phase distributes within the framework.
Investigations have shown that undercooling is favorable to refining the grains[12]. However, SEM micrographs show the grain size of Fe3B is coarser than that of Fe2B in the hyper-undercooled Fe83B17 alloy (Fig.3). This implies that there is a change under the hypercooling conditions compared with small under- coolings. According to classical nucleation theory, where the nucleation rate is governed by two exponential terms, i.e., the activation energy for the formation of critical nuclei and driving force for the atomic diffusion process. At small undercoolings, the exponential term containing activation energy for the formation of critical nuclei dominates, which will lead to the increase of nucleation rate with melt undercoolings whereas at high undercoolings, the exponential term containing driving force for atomic diffusion process dominates, which will result in the decrease of nucleation rates[13]. Whereas the growth velocity of nucleus is sufficiently high as a result of high driving force, i.e. high undercooling. Therefore, grain coarsening occurs.
3.2 Critical nucleation work
According to classical nucleation theory, critical nucleation work (ΔG*) is given by
![]() (2)
(2)
![]() (3)
(3)
![]() (4)
(4)
The parameters have been introduced particularly in Ref.[1]. It is known that critical nucleation work describes the extent of difficulty to nucleate. The interfacial energy (σsl) as one factor of the nucleation barrier depends on the structure of the nucleus. The Gibbs free energy difference ΔGv is often determined by using the linear approximation[14], which is driving forces for crystal nucleation in alloy melts and has relation to undercooling.
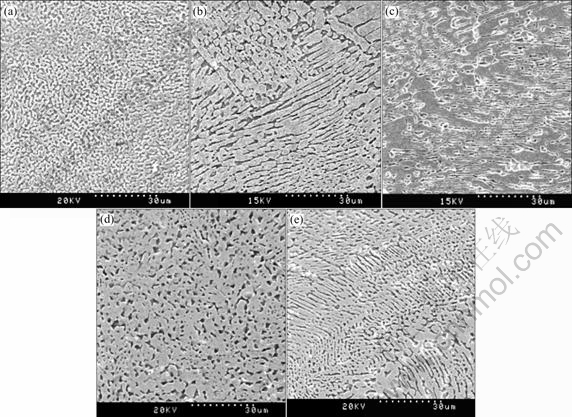
Fig.3 SEM micrographs of as-solidified Fe-B alloy samples for different undercooling levels, Fe83B17: (a) Anomalous eutectic α-Fe (dark)+Fe2B(grey); (b)-(d) Anomalous eutectic α-Fe(dark)+Fe3B(grey); Fe80B20 samples: (e) Anomalous eutectic α-Fe (dark)+Fe3B(grey); (a) ΔT=341 K; (b) ΔT=386 K; (c) ΔT=445 K; (d) ΔT=460 K; (e) ΔT=485 K
Table 1 Thermodynamic parameters of Fe83B17 alloy

A set of Fe83B17 alloy thermodynamic parameters selected for calculation is given in Table 1. The catalytic potency factor f (θ) was attained in the present work. The value of ΔG* was computed from Eqn.(2). The calculation result is shown in Fig.4. It is clear that the critical nucleation work of Fe3B phase and Fe2B phase decreases with increasing undercooling. That is to say the higher the undercooling is, the more unstable the melt is, which will result in a favorable energy condition to metastable phase formation. And when the undercooling of melt is larger than 273 K (the melt temperature is below about 1 160 K), the metastable phase (Fe3B) has less nucleation work in comparison with stable phase (Fe2B). So Fe3B phase will nucleate directly from the undercooled melt as a favorable phase. Though the calculation result of the critical undercooling (273 K) has some difference from the experimental data. This is understandable because the thermodynamic parameters are selected from several sources, it is possible there are some minute errors, for example, f (θ) is difficult to measure in experiment, here, f (θ) is considered a free para- meter and equal to both competitive phases[1]. On the other hand, the metastable phase may be decomposed at ambient temperature unless ΔT≥386 K, which was achieved in the experiments (Fig.2). Here, we only take into account competitive nucleation and ignore competitive growth principle, which is a possible reason that the theoretic value is small. Accordingly, competitive growth of metastable phase Fe3B and table phase Fe2B should also be considered when both competing phases share the same crystalline characteristics. That is to say not only competitive nucleation plays main role to control phase selection but also competitive growth plays considerable role, phase selection is controlled by coaction of two kinds of mechanism. It is required to ulteriorly investage the competitive growth mechanism of hypercooled Fe-B eutectic alloy.
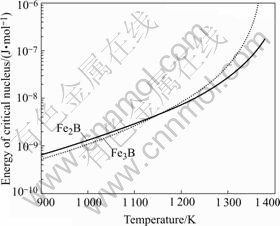
Fig.4 Steady-state critical nucleation work as function of temperature for Fe2B and Fe3B phases in undercooled Fe83B17 melt
4 Conclusions
In the undercooled melt, undercooling as a main factor determines the phase selection. Under the hypercooling condition, the hypercooling provides sufficient driving forces for the phase selection, and the favorable phase will form by competitive nucleation and growth. In present experiments a hypercooling was achieved, which suppressed the formation of stable phase Fe2B and accelerated the formation of metastable phase Fe3B in Fe-17%B and Fe-20%B alloys, respectively. And at room tempera- ture bulk metastable phase samples were obtained. TEM and XRD analyses and classical nucleation theory verified the relation between phase selection and undercooling.
References
[1] Herlach D M. Solidification from undercooled melts[J]. Mater Sci Eng A, 1997, A226-228: 348-356.
[2] Herlach D M, GAO J, Holland-Moritz D, et al. Nucleation and phase selection in undercooled melts[J]. Mater Sci Eng A, 2004, A375-377: 9-15.
[3] L?ser W, Hermann R, Lenonhardt M, et al. Metastable phase formation in undercooled near-eutectic Nb-Al alloys[J]. Mater Sci Eng A, 1997, A224: 53-60.
[4] Abbaschian R, Lipschutz M D. Eutectic solidification processing via bulk melt undercooling[J]. Mater Sci Eng A, 1997, A226: 13-21.
[5] Mleonhardt, L?ser W, Lindenkreuz H G. Solidification kinetics and phase formation of undercooled eutectic Ni-Nb melts[J]. Acta Mater, 1999, 47: 2961-2968.
[6] Battezzati L, Antonione C, Baricco M. Undercooling of Ni-B and Fe-B alloys and their metastable phase [J]. J Alloys Comp, 1997, 247: 164-147.
[7] DUHAI P, ?VEC P. Formation of metastable phase from amorphous state[J]. Mater Sci Eng A, 1997, A226-228: 245 -254.
[8] FUJITA H, Hashimoto K. Initial stages of crystallization in amorphous Fe-B and Ni-B alloys[J]. Mater Sci Eng A, 1980, 45: 221-228.
[9] Hallemans B, Wollants P, ROOS J R. Thermodynamic reassessment and calculation of the Fe-B phase diagram[J]. Z Metallkd, 1994, 85(10): 676-682.
[10] 36-1332, JCPDS [S]
[11] 39-1315, JCPDS [S].
[12] LI De-lin, YANG Gen-cang, ZHOU Yao-he. Grain refinement in bulk undercooled Ni-based alloy[J]. Trans Nonferrous Met Soc China, 1993, 3(4): 66-71.
[13] Herlach D M. Non-equilibrium solidification of undercooled metallic melts[J]. Mater Sci Eng R, 1994, R12: 252.
[14] Turnbull D. Formation of crystal nuclei in liquid metals[J]. J Appl Phys, 1950, 21: 1022.
[15] Palumbo M, Cacciamani G, BOSCO E, et al. Thermodynamic analysis of glass formation in Fe-B system[J]. CALPHAD, 2001, 25:625-637.
Foundation item: Projects(50395103; 50271057; 50501020) supported by the National Natural Science Foundation of China
Corresponding author: YANG Chang-lin; Tel: +86-29-88491484; E-mail: changlinyang@126.com
(Edited by LONG Huai-zhong)


