Trans. Nonferrous Met. Soc. China 23(2013) 1002-1010
Cavitation and electrochemical characteristics of thermal spray coating with sealing material
Seong-Jong KIM1, Seung-Jun LEE1, In-Ju KIM2, Seong-Kweon KIM3, Min-Su HAN1, Seok-Ki JANG1
1. Division of Marine Engineering, Mokpo Maritime University, Haeyangdaehak-ro 91 Mokpo-Si, 530-729, Korea;
2. Korea Institute of Industrial Technology, 89 Yangdaegiro-gil, Ipjang-myeon, Seobuk-gu, Cheonan-Si, 331-822, Korea;
3. Department of Electronic IT Media Engineering, Seoul National University of Science & Technology, 138 Gongneung-gil, Nowon-gu Seoul, 139-743, Korea
Received 9 April 2012; accepted 15 August 2012
Abstract:
Steel applied in ocean environment is exposed to corrosion and cavitation and is subject to increasing damages. To prevent this, anti-corrosion thermal spray coating technique is widely used. The low-temperature thermal spray coating was performed with 85%Al-14.5%Zn-0.5%Zr for ship materials and various sealing materials were applied to improve its durability, and the electrochemical behavior and cavitation characteristics were observed. The results show that the sealing improves all the properties of the materials. Hybrid ceramic and fluoro-silicon sealing materials show good electrochemical characteristics, and the fluoro-silicon sealing material shows the best anti-cavitation characteristics.
Key words:
thermal spray coating; sealing material; cavitation; electrochemical characteristics;
1 Introduction
Since metal corrosion is greatly influenced by flow speed and turbulence flow, that is, when flow speed increases, corrosion is accelerated remarkably [1,2]. Corrosion protection with sacrificial anode clearly decreases life span, and corrosion protection by impressed current tends to increase current density. In the case of sacrificial anode method, design techniques to predict life span are inadequate [3]. If sufficient corrosion protection cannot be achieved, painting must be used, but defective parts of painting exist [4,5]. Thus, corrosion begins from the defective parts and expands to the whole metal due to the defect of coating. As a technique to solve this problem, cold spray coatings which apply corrosion protection coating to metal surfaces were used in various industrial structures. Cold spray coating forms porous metal coating by a process that molten metal is sprayed out from a round slit, the distributed metal droplets hit the steel surface and are laminated and cooled. In the related studies, LIMA et al [6] investigated the fracture toughness and cavitation resistance of thermal spray coating through indentations and found a correlation between the two variables: the higher fracture toughness got, the better cavitation characteristics became. KIM et al [7] studied the void control, abrasion resistance and corrosion resistance of the ceramic spray layer of alumina. In this study, low-temperature spray coating technology with excellent electrochemical characteristics and its cavitation resistance in marine environment was incestigated. Spray coating with 85%Al-14.5%Zn- 0.5%Zr alloy wire for corrosion resistance was applied to steel which was used as a ship material, and the electrochemical and cavitation characteristics of sealing materials were compared.
2 Experimental
High tensile steel with composition of 0.1617% C, 0.013% Si, 0.659% Mn, 0.0146% P, 0.0076% S, balance Fe, was used and its tensile strength, yield strength, elongation are 463 MPa, 312 MPa and 23%, respectively. For thermal spray coating, KMS-300 arc spray was used to coat the steel in the thickness of 500 μm or higher with 85%Al-14.5%Zn-0.5%Zr alloy, spray transfer speed of 10 cm/s, gas pressure of 49-58.8 N and wire feed speed of 12 m/min. Thereafter, sealing materials such as water-soluble fluorine (hereinafter W-F), nano-fluorine (hereinafter nano), hybrid ceramic (hereinafter ceramic), salt-tolerant epoxy (hereinafter epoxy) and fluoro-silicon (hereinafter F-Si) were additionally applied [8]. In particular, F-Si has the adventages of long-term maintenance when applied to flooded and exposed areas of the marine environment under harsh conditions such as salt water, ultraviolet light and rain. In addition, the effect of adhesion for prevention of marine organisms such as barnacles is also excellent, and long-term protected by forming strong elastic film. The reference electrode and counter electrode were Ag/AgCl electrode and Pt electrode, respectively. All electrochemical experiments were conducted in natural circumstance (specific resistance 21.46 Ω·cm , pH 8.0, chloride concentration 16197×10-6, dissolved oxygen content 7.7×10-6). The multi-channel potentio/galvanostat WMPG-1000 was used for the corrosion test. The open circuit potential measurement was performed for 24 h to measure the potential behavior with time. Anodic and cathodic polarizations were tested from the open circuit potential to 3.0 V and -2.0 V at the scan rate of 2 mV/s. The corrosion potential and corrosion current density by Tafel analysis were obtained by polarizing ±0.25 V at the scan rate of 1 mV/s based on the open circuit potential. In accordance with the requirements of ASTM—G32 a ultrasonic vibration generator was used for the cavitation test, which generated a rated output of 20 kHz and supplied it to the vibrator; the distance of 1 mm was maintained using a filler gauge. An electronic balance that can measure down to 10-4 g was used to measure the mass before and after test. For microscopic analysis and evaluation after experiment, the corrosion pattern of the surface was observed with a 3D microscope.
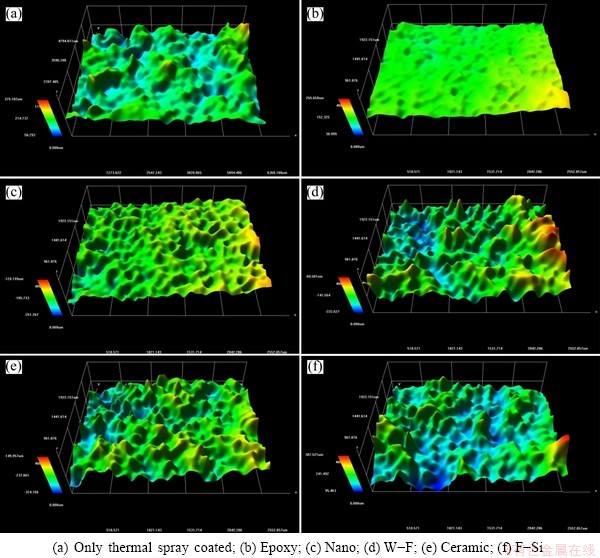
Fig. 1 Surface morphologies of 85%Al-14.5%Zn-0.5%Zr coating with various sealing materials
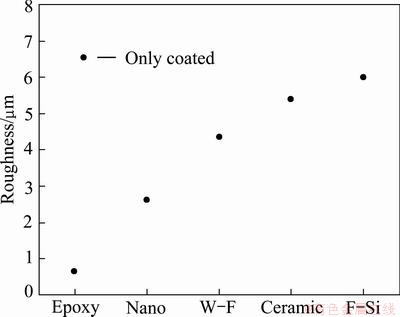
Fig. 2 Roughness of 85%Al-14.5%Zn-0.5%Zr coating with sealing materials
3 Results and discussion
Figures 1 and 2 show the changes of surface morphology by sealing material after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr alloy and compare the average roughness (Ra) using a roughness analysis program, respectively. The surface roughness Ra is the average of the absolute values from the center of height variation to the profile of the surface within the cutoff. The same cutoff was applied to all the specimens under different conditions to compare their measurements of average roughness. The unsealed surface of thermal spray coating layer was rough and many pores could be observed. The highest measured Ra was 6.895 μm. On the other hand, the specimen sealed with epoxy showed relatively very smooth surface and no surface defects or pores could be seen. The lowest measured Ra was 0.655 μm among the sealed specimens. That was expected to affect the corrosion characteristics. The surface sealed with nano sealing materials was little rougher than that with epoxy materials, with the Ra value of 2.614 μm, indicating a relatively even surface. The Ra values of W-F, ceramic, and F-Si sealing materials were 4.339, 5.378 and 5.979 μm, respectively. The surface morphologies showed rough surfaces similar to the base metal. When the Ra values in each condition were compared between different sealing materials after the thermal spray coating with 85%Al-14.5%Zn-0.5%Zr, the Ra value decreased by 90.5% for epoxy sealing material , 62.1% for the nano sealing material, 37.1% for the water-soluble fluorine, 22.0% for ceramic sealing material, and 13.3% for fluoro-silicon sealing material, over the base metal. As shown in Fig. 2, among the measured Ra values of various specimens, the epoxy sealing material was the best. The surface roughness affects the erosion and corrosion characteristics of the surface, thus epoxy and nano sealing materials which showed low roughness can offer better corrosion resistance than other materials.
Figure 3 compares the side observation results of different sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr. The coating layers were similar, ranging from 572.8 μm to 596.4 μm with a small difference of about 13 μm. This small difference is due to the irregular laminating process during the thermal spray. Since the same thermal spray was applied, the adhesiveness to the base metal will be similar. Furthermore, the coating layer was formed well with few pores in all conditions, but it still showed a little difference depending on the sealing material. No sealing material was observed in three specimens excluding those with epoxy and nano sealing materials, due to the fact that the sealing material was absorbed into the coating layer. The thicknesses of the epoxy and nano sealing materials were 256.8 μm and 130.5 μm, respectively, and they showed clear interface between the sealing layer and the thermal spray coating layer. Almost no defects were observed at the interfaces between the base metal and the coating layer and between the coating layer and the sealing layer, suggesting very sound bonding. On the other hand, a small number of micropores were observed inside the coating layer. These micropores of the coating layer could exhibit shock buffering effect or maximize corrosion resistance effect by inhibiting the generation of hydrogen in the coating that contacts solutions by electrically insulating the anode and cathode through sealing or painting. Even if the base metal was exposed to seawater due to defects caused by pores or oxide layer, the base metal electrochemically acted as cathode and the thermal spray coating layer acted as anode; and if micro-galvanic cells were formed, the thermal spray coating layer could play the role of sacrificial anode and will exhibit corrosion resistance effect for the base metal.
Figure 4 shows the open circuit potential measurements of the specimen in seawater for 86400 s with different sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr. For water- soluble sealing materials, the potential during the initial stage of immersion was -0.929 V, after which it alternated between rising and falling; the potential stabilized at -0.917 V after around 23000 s at the end of the experiment. For nano-fluorine sealing material, the potential was -0.915 V in the early stage of immersion and slowly moved toward the active direction; after around 15300 s, the potential remained at -0.92 V. At around 43000 s, the potential rose sharply and remained stable until the end of the experiment. For the hybrid ceramic sealing material, the potential was around -0.91 V in the early stage of immersion and rapidly moved toward the active direction from 24000 s to 48000 s. After this, the potential decreased slowly and indicated the active potential of -1.02 V at the end of the experiment. For the fluoro-silicon sealing material, the potential alternated between rising and falling, but it generally remained stable, and indicated -0.90 V at the end of the experiment. For the salt-tolerant epoxy sealing material, the potential was as low as -5.06 V in the early stage of immersion, and then steadily increased until 20000 s and remained constant after that. It showed the very noble potential of -0.56 V, due to the excellent environment-blocking effect of the compact coating layer. Therefore, we could not expect the effect of sacrificial anode from the salt-tolerant epoxy sealing material as it showed a higher potential than the base metal (KR-RA steel: -0.7 V). In the case of 85%Al-14.5%Zn-0.5%Zr which was coated before sealing, chlorine ions in the seawater penetrated into the voids in the coating layer with decreased potential [9]. As a result, it showed the potential of -0.979 V at the end of the experiment. After sealing with water-soluble fluorine, nano-fluorine, and fluoro-silicon, the potential became nobler than this which improved corrosion resistance. It might be because the sealing materials filled the voids and restricted the penetration of chlorine ions.

Fig. 3 Cross-sectional morphologies of 85%Al-14.5%Zn-0.5%Zr coating with different sealing materials

Fig. 4 Potential measurement of specimen in seawater with different sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr
Figure 5 presents the anodic polarization trend for different sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr. The open circuit potentials were similar in general, and a steady increase of current density was observed with increasing potential with no passive tendency in all conditions. However, the current density varied greatly as the potential increased. The salt-tolerant epoxy sealing material showed a very low current density compared with other conditions. The reason for this appeared to be that the electrochemical behavior is dependent on the characteristics of surface coating, and the coating layer has excellent environmental blocking effects as seen from the measurements of open circuit potential. The fluoro- silicon, hybrid ceramic, and water-soluble fluorine sealing materials showed similarly high current densities. Aluminum alloys in seawater environment maintain corrosion resistance to some degree due to the re-passivation, by which the passive films of Al2O3 or Al2O3·3H2O are destroyed or recreated by chlorine ions (Cl-) in seawater. However, no passivation was observed for the test materials in this study.
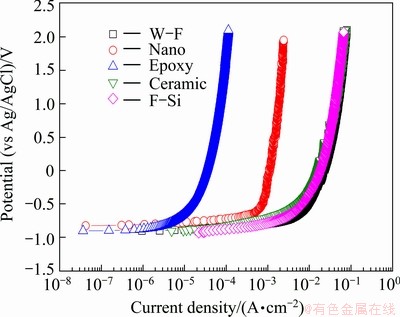
Fig. 5 Anodic polarization curves of specimen in seawater with sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr
Figure 6 depicts the cathodic polarization trend with different sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr alloy. The cathodic polarization of the metals immersed in seawater solution generally exhibits two distinct reactions: concentration polarization by the reduction of dissolved oxygen (O2+2H2O+4e→4OH-) and active polarization by hydrogen gas generation (2H2O+2e→H2+2OH-) [10-12]. For the water-soluble fluorine and fluoro- silicon sealing materials, however, concentration polarization and active polarization trends were not observed, and the current density slowly increased as the potential moved toward the active direction. The hybrid ceramic sealing material showed the trend of concentration polarization in the range from -1.884 V to -0.984 V, and the current density ranged from 1.015×10-6 A/cm2to 1.010×10-4 A/cm2. Besides, the nano-fluorine sealing material showed concentration polarization trend in the similar range (-2.057 to -1.030 V) as the hybrid ceramic sealing material, but its current density was little lower than that of the hybrid ceramic sealing material. The salt-tolerant epoxy sealing material most clearly showed the concentration polarization and active polarization trend, and the lowest current density of 10-6A/cm2 between -2.243 V and -0.979 V. The current density rose sharply at potential under -2.4 V, but it was considerably lower than the potential of other sealing materials. And the salt-tolerant epoxy sealing material showed a very low current density at potential under -2.4 V even though it was a hydrogen embrittlement section by the generation of hydrogen gas in almost all conditions. Thus, the salt-tolerant epoxy sealing material was also highly resistant to hydrogen embrittlement.
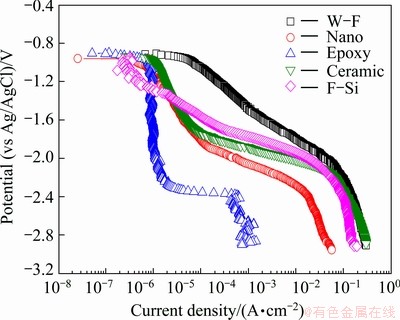
Fig. 6 Cathodic polarization curves of specimen in seawater with sealing materials after thermal spray coating with 85%Al- 14.5%Zn-0.5%Zr
Figure 7 shows the polarization curves of Tafel analysis in seawater with different sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr. For the water-soluble fluorine sealing material, the current density rose slowly in the cathodic polarization curve, and the current density increased steadily according to the rising potential in the anodic curve. When the current density was the highest among the applied sealing materials, its corrosion speed was the fastest in static condition. For the nano-fluorine sealing material, the corrosion potential was the lowest, and the pitting corrosion potential appeared at -0.897 V, at which the current density increased sharply. This trend was not observed in other sealing materials. The salt-tolerant epoxy sealing material showed low corrosion current density and high corrosion potential, which suggested excellent corrosion resistance. When the corrosion current density was compared, the water-soluble fluorine sealing material showed the highest value, whereas the nano-fluorine and salt-tolerant epoxy sealing materials showed considerably low corrosion current density, indicating a strong resistance to corrosion.
Figure 8 shows the observation results of the surface morphology according to cavitation time in various sealing materials and painting after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr. After about 10 min, only the painted specimen was damaged. At 20 min, the hybrid ceramic sealing material discolored over a wide area. For the nano-fluorine sealing material, the surface sealing was a little damaged. At 30 min, a damage was observed at the center of the hydrogen water-soluble sealing material, and the center of the salt-tolerant epoxy sealing material began to be damaged. At 60 min, a damage at the center of the hybrid ceramic sealing material specimen was observed, and the damages of all specimens began to accelerate from this moment. At 90 min, the base metal of the hybrid ceramic sealing material and the nano-fluorine sealing material began to be revealed. At 120 min, the base metals began to be revealed at the center of all specimens and the revealed area of the base metal increased over time.
Figure 9 shows the curves of mass loss and mass loss rate with cavitation time of different sealing materials and painting after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr. As shown in Fig. 9(a), the mass loss continuously increased over time for all the five sealing materials in all sections, and the mass loss rate of the painted materials steadily increased from the start to the end of the experiment. The nano-fluorine sealing material showed the lowest mass loss initially, but the mass loss increased over time and became greater than those of other sealing materials at the end of the experiment. The hybrid ceramic sealing material showed the greatest mass loss in the initial stage of experiment, but almost no increase of the mass loss was observed from 30 min to 90 min. Then, from 120 min, its mass loss increased slowly. The salt-tolerant epoxy sealing material showed relatively low mass loss in the early stage, but it increased sharply after 180 min. After 300 min, it showed the second highest mass loss after nano-fluorine sealing material. For water-soluble fluorine sealing material, the mass loss increased a little between 20 min and 30 min, and then it remained low. For the fluoro-silicon sealing material, the mass loss increased a little in the first 20 min and sharply increased at 30 min. After 90 min, the mass loss increased steadily, indicating the lowest mass loss. The specimens that were sealed after thermal stray coating showed a mass loss 1/5 to 1/3 times lower than those of the specimens painted at the end of the experiment, giving improved anti-cavitation characteristic. The consumption rates by cavitation over time were compared. The painted material showed much higher mass loss rate than other sealing materials from the start of experiment. The hybrid ceramic sealing material showed a consumption rate that was 2.5-7 times higher than other sealing materials in the early stage of experiment, and the consumption rate sharply decreased after that and stabilized after 60 min. While the nano-fluorine sealing material exhibited the lowest consumption rate in the early stage, but it increased until at 60 min of experimental time and maintained a higher consumption rate than other sealing materials. The salt-tolerant epoxy sealing material alternated between rising and falling after a high consumption rate in the early stage, and after 60 min it became generally stabilized. The water-soluble sealing material showed a small width of change and the consumption rate decreased a little after 180 min. The fluoro-silicon sealing material showed a high consumption rate at 10 min and it decreases by half after 20 min and rapidly increased until 30 min. After that, the consumption rate sharply decreased until 60 min and stabilized after 90 min. Consequently, the cavitation consumption rate considerably improved compared with the painted case. The fluoro-silicon showed the lowest consumption rate, indicating the best resistance to cavitation.
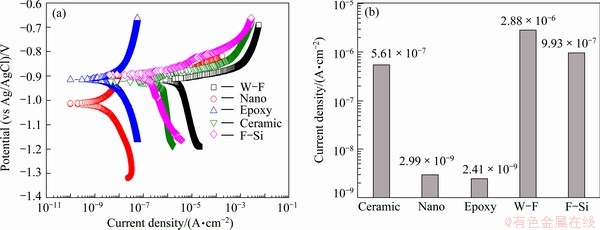
Fig. 7 Polarization curves for Tafel analysis (a) and corrosion current density (b) in seawater with different sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr

Fig. 8 Surface morphologies after cavitation test with different sealing materials and painting after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr

Fig. 9 Mass (a) and mass loss rate (b) after cavitation test in seawater with sealing materials and painting after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr
Figures 10 and 11 show the morphologies of damaged surfaces and the maximum damage depth taken with a 3D microscope after the cavitation experiment for specimens coated with various sealing materials, respectively. The ceramic sealing material showed steady damages starting with 117 μm from 10 min to 60 min, and a relatively large damage was observed at the center of specimen after 20 min of the experiment time. The damage progressed in the depth direction and the maximum depth steadily increased until 60 min. After that, the damages in the edges accelerated more than in the center and the depth began to be relatively shallower. Pitting increased again in the depth direction at 120 min and the largest depth of damage of 399 μm appeared at 180 min. At 300 min, the coating layer in the edges with relatively poor erosion resistance began to be damaged and a shallow damage depth of 95 μm was observed. The nano sealing material showed the lowest damage depth among the five sealing materials, and the greatest damage depth of 200 μm was observed at just 20 min. The damage depth became gradually lower and decreased to 30-80 μm after 60 min. The epoxy sealing material showed rough surface morphology due to pitting at 20 min. After that, pits were combined to increase damages and the surface became flat. As the damages accelerated in the depth direction, most of the base metal was revealed at 180 min. For the water-soluble fluorine sealing material, a large pitting with the depth of 144 μm occurred at just 10 min, and damages by pitting corrosion progressed at 20 min, but the maximum damage depth did not increase. And the surroundings became flat at 30 min and damages occurred again in the depth direction, reaching the maximum damage depth of 223 μm. After that, the depth became lower from 60 min to 90 min as nearby pits were combined due to continued shock which flattened the surface. At 120 min, the pit at the center grew in the depth direction and reached the maximum depth of 354 μm. The microscope pictures also showed that a large part of the base metal at the center began to be revealed. At 300 min, the damage depth decreased to 75 μm. The reason for the decreased damage depth is that even though the base metal was partially damaged, the base metal was exposed due to the damage of the surrounding coating layer with relatively poor erosion resistance. For the fluoro-silicon sealing material, a damage with the depth of 615 μm was observed at 60 min. The reason for this is that a pitting developed at the center of the specimen, and its growth in the depth direction accelerated until 60 min. The pictures also show that the pitting is larger and the peeled surrounding coating layer progressed more at 60 min than at 20 min. In addition, at 90 min and 120 min, the surrounding coating layer was damaged and flattened with the growth of the pitting, which decreased the maximum damage depth. At 180 min, the peeling speed of the surrounding coating layer was faster than the growth of the center pitting in the depth direction, and as the base metal was revealed, the damage depth increased to 609 μm. At 300 min, the damage depth decreased because the erosion progressed more in the surrounding area with relatively low strength due to the exposed base metal. The pictures of the damaged surface and the graph of the measurements of the maximum damage depth do not agree with the mass loss and cavitation rate in Fig. 9 because the exposed time is different due to the difference in the resistance to cavitation of the coating layer.

Fig. 10 Surface morphologies of specimen after cavitation test in seawater with sealing materials after thermal spray coating with 85%Al-14.5%Zn-0.5%Zr

Fig. 11 Maximum damage depth taken with 3D microscope after cavitation experiment
4 Conclusions
1) The electrochemical experiment shows that the salt-tolerant epoxy sealing material has excellent corrosion resistance, and no sacrificial anode effect can be expected due to a higher potential than that of the base metal.
2) The water-soluble fluorine sealing material shows the highest corrosion current density, indicating weak corrosion resistance. And the nano-fluorine sealing material shows a considerably low corrosion current density, but it shows the highest mass loss, indicating poor anti-cavitation characteristic.
3) In general, the use of sealing materials greatly increases the resistance to cavitation. Overall, the fluoro- silicon sealing material exhibits the best anti-cavitation characteristics, so it is believed to be the best sealing material in cavitation environment.
References
[1] SUN Z, KANG X Q, WANG X H. Experimental system of cavitation erosion with water-jet [J]. Materials and Design, 2005, 26: 59-63.
[2] HEITZ E. Chemo-mechanical effects of flow on corrosion [J]. Corrosion, 1991, 47: 135-145.
[3] JEON D H, KIM W Y. A study on the cathodic protection of a steel pipe in water by impressed current method [J]. Journal of Corrosion Science Society of Korea, 1979, 8(1): 3-16.
[4] ZHU X, GE Y, GE R. Effect of sacrificial anode protection for steel pipe piles at Dandong port [J]. China Ocean Engineering, 1998, 12(1): 113-120.
[5] WANG H, HU S, YANG S. The analogue test research of the sacrificial anode protection at a seawater cooller [J]. Corrosion Science and Protection Technology, 1997, 9(4): 63-66.
[6] LIMA M M, GODOY C, MODENESI P J, AVELAR-BATISTA J C, DAVISON A, MATTHEWS A. Coating fracture toughness determined by Vickers indentation: An important parameter in cavitation erosion resistance of WC-Co thermally sprayed coatings [J]. Surf Coat Tech, 2004, 177-178: 489-496.
[7] KIM Y S, KIM Y H, KIM J H, CHOI Y G, KANG T Y. A study on the evaluation of the friction and wear properties of the sprayed coating layer [J]. The Korean Welding and Joining Society, 1996, 14(3): 66-74.
[8] HAN M S, LEE S J, JANG S K, KIM S J. Electrochemical and cavitation characteristics of Al thermal spray coating with F-Si sealing [J]. Corrosion Science and Technology, 2010, 9(6): 317-324.
[9] ARAMAKI K, SHIMURA T. Prevention of passive film breakdown and corrosion of iron in 0.1M KClO4 with and without Cl- by covering with an ultrathin two-dimensional polymer coating and healing treatment in 0.1M NaNO3 [J]. Corrosion Science, 2010, 52: 2766-2772.
[10] KIM S J, JANG S K, KIM J I. Effects of post-weld heat treatment on optimum cathodic protection potential of high-strength steel in marine environment conditions [J]. Materials Science Forum, 2005, 486-487: 133-136.
[11] KIM S J, JANG S K, KIM J I. Characteristics evaluation of thin films formed in Mg-Al alloy in various chemical conversion solution conditions [J]. Journal of the Korean Society of Marine Engineers, 2005, 29(1): 98-106.
[12] KIM S J, KIM J I. Evaluating the electrochemical properties in the protection potential of material for use in Al vessels in seawater [J]. Materials Science Forum, 2006, 510-511: 158-161.
采用密封材料的热喷涂层的空蚀和电化学特征
Seong-Jong KIM1, Seung-Jun LEE1, In-Ju KIM2, Seong-Kweon KIM3, Min-Su HAN1, Seok-Ki JANG1
1. Division of Marine Engineering, Mokpo Maritime University, Haeyangdaehak-ro 91 Mokpo-Si, 530-729, Korea;
2. Korea Institute of Industrial Technology, 89 Yangdaegiro-gil, Ipjang-myeon, Seobuk-gu, Cheonan-Si, 331-822, Korea;
3. Department of Electronic IT Media Engineering, Seoul National University of Science & Technology, 138 Gongneung-gil, Nowon-gu Seoul, 139-743, Korea
摘 要:抗腐蚀热喷涂技术广泛地运用于防止钢在海洋环境中由于腐蚀和气蚀受到损害。为了改善用于船舶材料的低温热喷涂85%Al-14.5%Zn-0.5%Zr涂层合金的耐久性,对其采用了多种密封材料,并考察其电化学行为和空蚀特征。结果表明:密封材料能改善涂层材料的性能。其中,混合陶瓷和氟-硅密封材料有着良好的电化学性能,并且氟-硅密封材料具有最佳的抗空蚀性能。
关键词:热喷涂层;密封材料;空蚀;电化学性能
(Edited by Xiang-qun LI)
Foundation item: Project supported by the Ministry of Education, Science Technology (MEST) and Korea Industrial Technology Foundation (KOTEF) through the Human Resource Training Project for Regional Innovation
Corresponding author: Seung-Jun LEE; E-mail: corr-pro@mmu.ac.kr; Seong-Kweon KIM; E-mail: kim12632@seoultech.ac.kr
DOI: 10.1016/S1003-6326(13)62559-5
Abstract: Steel applied in ocean environment is exposed to corrosion and cavitation and is subject to increasing damages. To prevent this, anti-corrosion thermal spray coating technique is widely used. The low-temperature thermal spray coating was performed with 85%Al-14.5%Zn-0.5%Zr for ship materials and various sealing materials were applied to improve its durability, and the electrochemical behavior and cavitation characteristics were observed. The results show that the sealing improves all the properties of the materials. Hybrid ceramic and fluoro-silicon sealing materials show good electrochemical characteristics, and the fluoro-silicon sealing material shows the best anti-cavitation characteristics.


