Trans. Nonferrous Met. Soc. China 25(2015) 4175-4182
Reductive acid leaching of cadmium from zinc neutral leaching residue using hydrazine sulfate
Chun ZHANG1,2, Xiao-bo MIN1,3, Jian-qiang ZHANG1, Mi WANG1, Bo-sheng ZHOU1, Chen SHEN1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Municipal and Mapping Engineering, Hunan City University, Yiyang 413000, China;
3. Chinese National Engineering Research Center for Control & Treatment of Heavy Metal Pollution, Central South University, Changsha 410083, China
Received 28 January 2015; accepted 24 June 2015
Abstract:
Zinc neutral leaching residue (ZNLR) from hydrometallurgical zinc smelting processing can be determined as hazardous intermediate containing considerable amounts of Cd and Zn which have great threats to the environment. The ZNLR contained approximately 35.99% Zn, 15.93% Fe and 0.26% Cd, and Cd mainly existed as ferrites in the ZNLR in this research. Reductive acid leaching of ZNLR was investigated. The effects of hydrazine sulfate concentration, initial sulfuric acid concentration, temperature, duration and liquid-to-solid ratio on the extraction of Cd, Zn and Fe were examined. The extraction efficiencies of Cd, Zn and Fe reached 90.81%, 95.83% and 94.19%, respectively when the leaching parameters were fixed as follows: hydrazine sulfate concentration, 33.3 g/L; sulfuric acid concentration, 80 g/L; temperature, 95 °C; duration of leaching, 120 min; liquid-to-solid ratio, 10 mL/g and agitation, 400 r/min. XRD and SEM-EDS analyses of the leaching residue confirmed that lead sulfate (PbSO4) and hydrazinium zinc sulfate((N2H5)2Zn(SO4)2) were the main phases remaining in the reductive leaching residue.
Key words:
reductive acid leaching; zinc neutral leaching residue; hydrazine sulfate; cadmium;
1 Introduction
Cadmium is one of the toxic metals, causing chronic poisoning through inhalation and ingestion and having serious potential ecological risk [1], which is primarily produced as byproduct from mining, smelting, and refining of zinc sulfide concentrate, as it is naturally associated with zinc sulfide ore, typically from 0.5% to 1.5% [2]. However, cadmium is an important metal, which finds applications in production of various materials such as alloys, batteries, ceramics, pigments and metal plating [3]. Large scale of wastes containing cadmium in the form of slag, sludge, smoke and wastewater were discharged into the environment when cadmium-containing materials were developed and utilized in the industries, especially in the non-ferrous smelting industry. For instance, according to the reports, the average Cd emission factors from artisanal zinc smelting using indigenous method in Hezhang, Guizhou, China, were 1460 and 1240 g Cd for one ton of Zn production produced from zinc sulfide and oxide ore,respectively [4]. With an ever increasing focus on the sustainable use of resources and environment protection, interest in the processing of secondary feed material sources as well as metal containing residues has substantially increased.
Because of relatively high iron content in the zinc sulfide ore, a significant part of zinc unavoidably converted into zinc ferrite [5,6] and simultaneously some zinc ions in the matrix of zinc ferrite might be substituted by the cadmium partly at high temperature. Therefore, the cadmium bearing zinc ferrite was formed during the roasting stage [2]. The sparingly soluble zinc ferrite and cadmium-bearing zinc ferrite makes it difficult for zinc and cadmium to be extracted during the neutral leaching of zinc calcine and thus the ferrites mainly remain in the ZNLR which creates problems in zinc and cadmium recovery by hydrometallurgical processes [7-9]. Thus, cadmium would disperse in leaching residue, purification residue, beta cake, pot skimming, etc, which were generated in traditional hydrometallurgical processing of zinc sulfide concentrate, therefore, increasing the diffusion frequency of cadmium.
In fact, both pyrometallurgy and hydrometallurgy routes were applied in treatment of such materials for the sake of recovering valuable metals at present. The application and development of pyromrtallurgy are, however, impeded by some of drawbacks like high energy and resource consumption, expensive operating costs and serious air pollution [10-12]. Similarly, the problems including the cumbersome flowsheet and complicated operations occurred when the method of hot concentrated acid leaching or pressure leaching followed by jarosite, goethite or hematite processes was applied [13].
Recently, a series of hydrometallurgical processes of high pressure acid leaching and the leaching use of various lixiviants of materials containing ferrites have been investigated focusing on recovery of zinc and more than 90% of zinc can be extracted out, but less attention has been taken to the leaching behavior of cadmium [14-16]. And simultaneously, citric acid, oxalic acid, sulfur dioxide and hydrogen peroxide [17-21] had been chosen as reductant in leaching various materials by researchers for acquiring a relatively mild leaching condition and high metal extraction efficiency.
Due to its strong reductive ability, hydrazine sulfate was chosen as reductant in leaching procedure for improving the decomposition efficiency of ferrites in dilute acid solution in this work. Aiming at extracting cadmium in the first leaching stage and restraining its diffusion during following operating units, valuable non-ferrous metals can be retrieved from this non-ferrous metals-enriched solution which can be further processed by the established procedure. The main reactions can be described as follows.
MeO+2H+→Me2++H2O (1)
MeO·Fe2O3+8H+→Me2++2Fe3++4H2O (2)
N2H5++Fe3+→NH4++1/2N2↑+H++Fe2+ (3)
where Me represents metals such as zinc, cadmium, cobalt and nickel.
The purpose of this research was to present the reductive extraction process of ZNLR using the mixed system of dilute sulfuric acid and hydrazine sulfate. The extraction of cadmium as well as zinc and iron from ZNLR was demonstrated. The effects of hydrazine sulfate concentration, sulfuric acid concentration, leaching temperature, leaching time and liquid-to-solid ratio were investigated.
2 Experimental
2.1 Materials
A 5 kg sample of ZNLR was collected from Shuikoushan Nonferrous Metals Group Co., Ltd. (SKS) located in Hunan Province, China. All the samples in this work were dried, ground and sieved with size less than 75 μm and above silica gel was stored in a desiccator. Extraction efficiencies of metals depend greatly on the mineralogical phase composition in the residue [22]. Therefore, in order to characterize the ZNLR, the sample was submitted to chemical and mineralogical analyses by chemical analysis, inductively coupled plasma-atomic emission spectroscopy (ICP-AES, VG PQEXCELL, Thermo Electron Corporation, USA), X-ray diffraction (XRD, Rigaku, TTR-III, Japan), and scanning electron microscopy with energy-dispersive spectrometry (SEM-EDS, Nova Nano SEM 230, USA).
2.2 Experimental setup and procedure
Batch leaching tests were performed in a 0.5 L three-neck flask placed in an isothermal water bath controlled by a thermostat to maintain the reactor temperature within ±1 °C. A mechanical stirrer was provided to keep good contact between the solution and the solid particles, a water-cooled condenser to avoid solution loss by evaporation and a thermometer to measure the temperature during the leaching experiment. But when the temperature was higher than 100 °C, the autoclave was used. All the chemicals employed in the study were of analytical grade.
The effects of various parameters such as hydrazine sulfate concentration, sulfuric acid concentration, leaching temperature, duration time and liquid-to-solid ratio on the leaching efficiency of metals were investigated. The agitation speed was fixed at 400 r/min in order to sufficiently suspend the slag and mix the contents of the reactor providing perfect hydrodynamic conditions for chemical processes.
The leach liquor was collected and filtrated, and the liquor sample was analyzed for zinc, iron and cadmium by EDTA-titration, K2Cr2O7-titration and ICP-AES, respectively. The leaching residue was washed and dried for X-ray diffraction and SEM-EDS analyses. The extraction of metal was calculated according to Eq. (4).
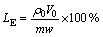 (4)
(4)
where LE is the metal leaching efficiency (%); ρ0 and V0 are the mass concentration of metal (g/L) in the leach liquor and the volume of leach liquor (L), respectively; m is the mass of ZNLR (g); and w is the mass fraction of the metal in ZNLR (%).
3 Results and discussion
3.1 Characterization of ZNLR
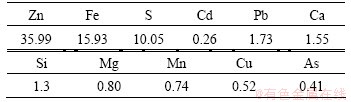
Results from chemical analysis of ZNLR are presented in Table 1. It can be observed that ZNLR gets a relatively high contents of Zn and Fe. Simultaneously, it also presents some other very toxic heavy metals such as Pb and As.
Table 1 Chemical composition of ZNLR (mass fraction, %)
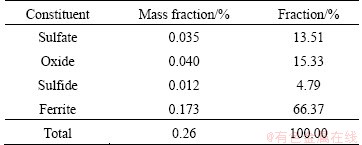
The XRD pattern of ZNLR shown in Fig. 1 indicates that the main phases present in the residue are zinc ferrite (ZnFe2O4) and zinc oxide (ZnO), as well as zinc sulfide (ZnS), zinc silicate (Zn2SiO4), zinc sulfate monohydrate (ZnSO4·H2O), zinc sulfate hydroxide hydrate (Zn4SO4(OH)6·H2O) and lead sulfate(PbSO4), which are similar to those reported by LI et al [23]. No special cadmium bearing minerals were identified because of its low content. The phase analysis of zinc in ZNLR is summarized in Table 2, based on the chemical analysis. Zinc oxide and zinc ferrite were the main phases and accounted for 11.20% and 10.77%, respectively, and others were zinc sulfate, zinc sulfide and zinc silicate phases which were in accordance with the XRD analysis. The cadmium present in the different phases of ZNLR is given in Table 3. Approximately 66.37% of cadmium exists in the form of ferrite.
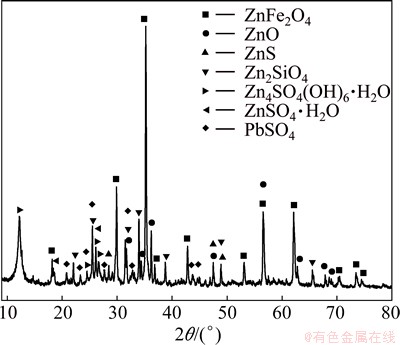
Fig. 1 XRD pattern of ZNLR
Table 2 Phase composition of zinc in ZNLR
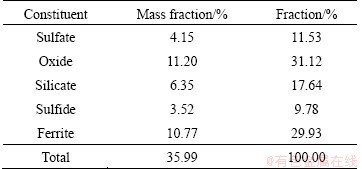
Table 3 Phase composition of cadmium in ZNLR
SEM-EDS analysis of ZNLR was performed and the image is shown in Fig. 2. Based on this microphotograph, it can be seen that the particle size of ZNLR is not homogeneous and generally in irregular bulk shapes. According to the EDS analysis, the particles as shown in Figs. 2(b) and (c) are of the similar chemical properties containing Cd, Zn and Fe simultaneously which indicates that most of the particles in ZNLR are agglomerate blocks formed during roasting which may increase the difficulty in leaching process due to the decreasing of effective contact area.
3.2 Effects of parameters
3.2.1 Effect of hydrazine sulfate concentration
The influence of hydrazine sulfate concentration on metals leaching efficiency was examined using five different hydrazine sulfate concentrations: 29.6, 33.3, 37.0, 40.7, 44.4 g/L and other parameters were fixed as sulfuric acid 80 g/L, leaching temperature 95°C, leaching time 120 min and liquid-to-solid ratio 10 mL/g based on previous studies. A contrast experiment was undertaken using plain sulfuric acid solution (80 g/L) with other parameters kept constant and the results were shown in Fig. 3. Based on Fig. 3, the extraction of metals in dilute sulfuric acid solution was relatively low. After leaching 120 min using sulfuric acid only, 42.28% of Cd and 64.02% of Zn were extracted, respectively, which were due to the dissolution of oxides, sulfates and silicates and 3.14% of Fe because of the insolubility of ferrites in dilute acid solution. Further increase in the leaching time had no significant effects.
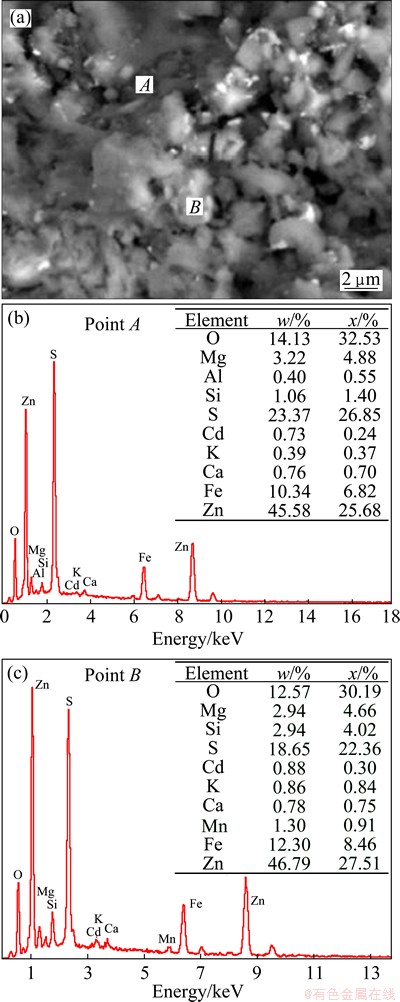
Fig. 2 SEM image (a) and EDS patterns (b,c) of ZNLR
The effect of hydrazine sulfate concentration on metals extraction efficiency from ZNLR is shown in Fig. 4. The results indicated that the extraction of zinc was generally higher than 90% within the range of the investigated hydrazine sulfate concentration and the leaching efficiency of Fe generally increased with the increase of the amount of hydrazine sulfate. However, leaching efficiency of Cd increased when the hydrazine sulfate concentration increased from 29.6 to 33.3 g/L and declined slightly with further increase of hydrazine sulfate concentration from 33.3 to 44.4 g/L and got the highest extraction efficiency when the hydrazine sulfate concentration reached 33.3 g/L where the extraction efficiency of Cd was 90.81%. Based on Fig. 4, after leaching 120 min, 90.81% of Cd, 95.53% of Zn and 94.19% of Fe were leached out, which were improved greatly compared with leaching using sulfuric acid only and suggested that the addition of hydrazine sulfate intensified the decomposition of the refractory ferrites.
3.2.2 Effect of sulfuric acid concentration
The dependence between the concentration of sulfuric acid and the leaching efficiency of metals was studied in this connection. Five tests were carried out at different initial sulfuric acid concentrations ranging from 35 g/L to 95 g/L for 33.3 g/L hydrazine sulfate concentration and liquid-to-solid ratio of 10 mL/g at 95 °C for 120 mim. As seen from Fig. 5, sulfuric acid concentration had significant effects on extraction of Cd, Zn and Fe, especially Fe. Generally, the leaching efficiencies of metals increased with the increase of sulfuric acid concentration, because of dissolving of metals in high acid concentration [24]. The curves of leaching efficiency of Cd, Zn and Fe increased fast with the increase in the sulfuric acid concentration from 35 to 80 g/L, but slowed down and remained unchanged when the concentration of sulfuric acid was further increased to 95 g/L. The extraction of Zn was faster than that of Cd and Fe when the sulfuric acid concentration was lower than 80 g/L which can be explained by the composition of mineral phase in ZNLR and its acidic dissolubility. The extraction efficiency of Cd, Zn and Fe reached 90.81%, 95.83% and 94.19%, respectively when sulfuric acid concentration was 80 g/L.
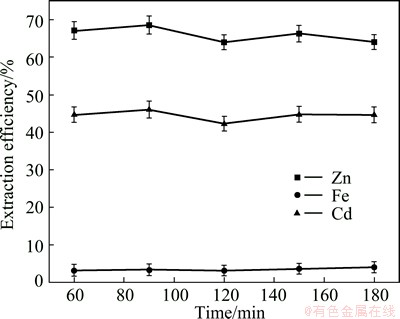
Fig. 3 Effect of time on metals extraction from ZNLR using sulfuric acid
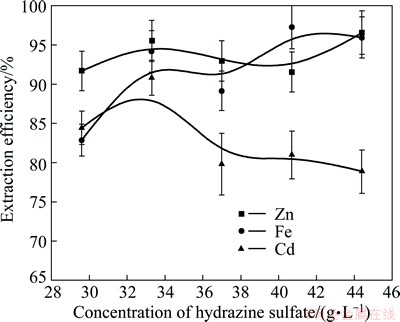
Fig. 4 Effect of hydrazine sulfate concentration on metals extraction from ZNLR

Fig. 5 Effect of sulfuric acid concentration on metals extraction from ZNLR
Figure 6 shows the XRD patterns of leaching residues from ZNLR leached by different concentrations of sulfuric acid solution. The peaks of zinc ferrite broadened and disappeared gradually with the increase in sulfuric acid from 35 to 95 g/L and disappeared completely at the concentration of 95 g/L, which distinctly indicated that increasing the concentration of sulfuric acid intensively accelerated the decomposition rate of ferrites.
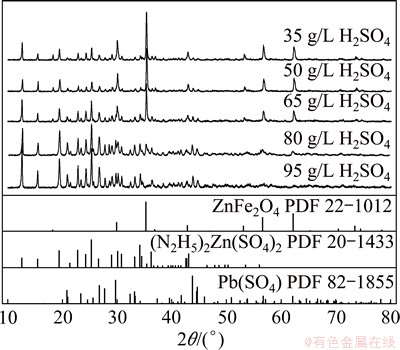
Fig. 6 XRD patterns of leaching residues from ZNLR leached by different concentrations of sulfuric acid
3.2.3 Effect of temperature
The extraction of metals is also significantly affected by leaching temperature. High metal extraction may not appear at a low temperature while high temperatures have disadvantages such as higher energy consumption. So, in this work, a comparatively low temperature range from 50 °C to 110 °C was selected and its effects on extraction of metals were evaluated. Materials were fixed as follows: hydrazine sulfate concentration, 33.3 g/L; sulfuric acid concentration, 80 g/L; leaching time, 120 min; and liquid-to-solid ratio, 10 mL/g. Results, as presented in Fig. 7, indicated that temperature had prominent effects on extraction of metals which was in accordance with the kinetic of acid leaching of ferrites [7,25]. The extraction of Cd increased from 43.19% to 90.81% when the temperature increased from 50 °C to 95 °C, but slowed down and remained unchanged with a further increase in temperature to 110 °C. Accordingly, the extraction of Zn increased from 69.08% to 95.83%, while that of Fe increased from 25.11% to 94.19% when the temperature increased from 50 °C to 95 °C and a further increase in temperature to 110 °C had no significant effects.

Fig. 7 Effect of leaching temperature on metals extraction from ZNLR
The XRD patterns of leaching residues shown in Fig. 8 suggested that the zinc ferrite in the ZNLR was decomposed almost completely as the temperature increased to 95 °C. The decomposition rate of zinc ferrite had been enhanced significantly by increasing the temperature due to the cause of dynamics. Therefore, the optimum temperature appeared to be 95 °C and all further experiments were carried out at this temperature considering the unreality of the temperature over the boiling point of water in practice.

Fig. 8 XRD patterns of leaching residues from ZNLR leached at different temperatures
3.2.4 Effect of time
A series of leaching experiments were conducted to estimate effects of residence time (from 30 to 240 min) on metals extraction efficiency. Materials were fixed as follows: hydrazine sulfate concentration, 33.3 g/L; sulfuric acid concentration, 80 g/L; leaching temperature, 95 °C and liquid-to-solid ratio, 10 mL/g. Figure 9 shows the variation in extraction of Cd, Zn and Fe as a function of leaching time. Generally, the extraction efficiencies of Cd, Zn and Fe increased with the increase of duration of leaching and Zn got higher extraction efficiency than Cd and Fe at the beginning due to the easier dissolution of Zn than Cd and Fe in this case. The extraction efficiency of Cd increased from 74.90% to 90.81% when the duration of leaching increased from 30 min to 120 min, but stayed the same when the leaching time lengthened from 120 min to 240 min. Besides, the extraction efficiency of Zn increased from 91.25% to 95.83% and that of Fe increased from 76.90% to 94.19% with the increase of leaching time from 30 min to 120 min, but a further increase in duration of leaching from 120 min to 240 min had no marked influences. The extraction efficiencies of Cd, Zn and Fe reached 90.81%, 95.83% and 94.19%, respectively after leaching 120 min while the residence time of hot acid leaching of zinc ferrite generally exceeded 4 h and sometime can reach up to 12 h because of a slow process of decomposition rate of zinc ferrite in hot acid leaching [26,27]. Considering the aforementioned results, the most favorable leaching time was fixed as 120 min.
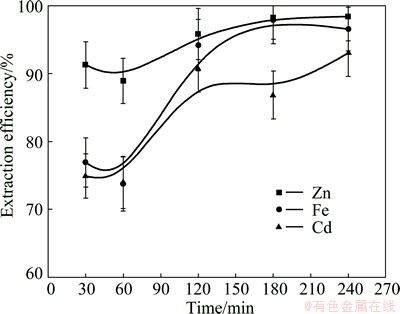
Fig. 9 Effect of leaching time on metals extraction from ZNLR
3.2.5 Effect of liquid-to-solid ratio
Figure 10 presents the effects of the relationship between the volume of the leaching agent and the ZNLR mass (L/S) on the leaching yield of Cd, Zn and Fe. For the liquid-to-solid ratio of 7 mL/g, the degrees of leaching of Cd, Zn and Fe compounds are 65.12%, 79.74% and 48.65%, respectively. A much greater leaching yield of Cd, Zn and Fe was achieved by increasing the L/S to a level of 10 mL/g where the extraction efficiencies of Cd, Zn and Fe reached 90.81%, 95.83% and 94.19% respectively because of the increase in amount of lixiviant. However, a further increase of L/S to 11 mL/g did not have significant effects on leaching efficiencies of metals.
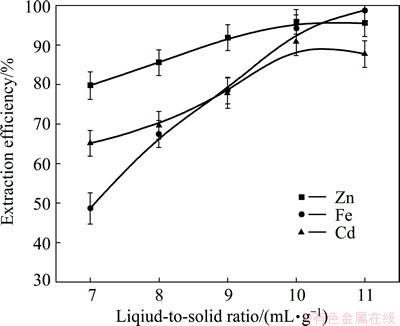
Fig. 10 Effect of liquid-to-solid ratio on metals extraction from ZNLR
The leaching parameters were fixed as follows: hydrazine sulfate concentration, 33.3 g/L; sulfuric acid concentration, 80 g/L; leaching temperature, 95 °C; duration of leaching, 120 min; and liquid-to-solid ratio range from 7 to 11 mL/g.
3.3 Characterization of leaching residue
The leaching residue, obtained under the optimum leaching conditions (hydrazine sulfate concentration, 33.3 g/L; sulfuric acid concentration, 80 g/L; leaching temperature, 95 °C; leaching time, 120 min; liquid-to- solid ratio, 10 mL/g and the agitation speed, 400 r/min) was submitted to SEM-EDS analysis. The XRD was selected to characterize the leaching residues obtained at different leaching time.
As shown in Fig. 11, the characteristic peaks of zinc ferrite weakened and disappeared gradually with time and the phases of lead sulfate (PbSO4) and hydrazinium zinc sulfate ((N2H5)2Zn(SO4)2) occurred simultaneously. And the phases of zinc in oxide, sulfide, silicate and sulfate were not found yet after 30 min leaching, attributing to their rapidly dissolving dynamics. Given the similarity in chemical properties between Zn and Cd, hydrazinium cadmium sulfate ((N2H5)2Cd(SO4)2) may be formed with the increase of hydrazine sulfate concentration during the leaching process which may not be determined by the XRD analysis because of the minor amount. That might be the reason why the extraction efficiency of cadmium declined slightly with further increase of hydrazine sulfate concentration as shown in Fig. 4.
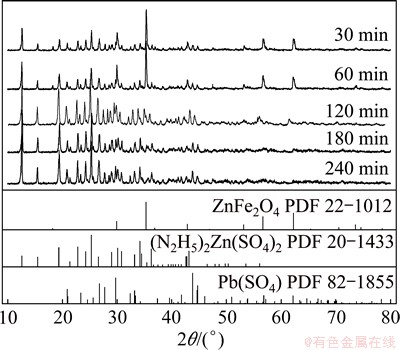
Fig. 11 XRD patterns of residue from ZNLR leached at different time
Figure 12 shows the SEM-EDS analysis of the leaching residue from ZNLR, the white particle is PbSO4 as shown in Fig. 12(a), which is consistent with the XRD analysis and the dark particles rich in PbSO4 as shown in Fig. 12(b) which contain minor amounts of other elements like Zn, Fe, Cu and Si may be the agglomerate blocks, which indicates that the element in agglomerate blocks particles would restraint the extraction of metals during leaching stage and might be dissolved by ball-milling before leaching stage.
4 Conclusions
1) It was observed that the addition of hydrazine sulfate as reductant in sulfuric acid leaching of Cd from ZNLR containing zinc ferrite and cadmium bearing zinc ferrite can greatly promote the extraction of Cd, as well as Zn and Fe, in a condition of comparatively low sulfuric acid concentration and leaching temperature.
2) The optimum leaching parameters in this work were determined as follows: hydrazine sulfate concentration 33.3 g/L, sulfuric acid concentration 80 g/L, leaching temperature 95 °C, leaching time 120 min and liquid-to-solid ratio 10 mL/g, and the agitation speed was fixed at 400 r/min. Under these conditions, 90.81% of Cd, 95.83% of Zn and 94.19% of Fe were extracted, respectively.
3) The phases of Zn in the form of oxide, sulfide, silicate and sulfate were easily extracted out and dissolved mostly during the first stage of leaching. The leaching of ferrites was slowly and significantly affected by the temperature and initial acid concentration. The phase of PbSO4 was not leached out and still remained in the leaching residue and the new phase hydrazinium zinc sulfate ((N2H5)2Zn(SO4)2) occurred during the process, suggesting that the appropriate control of hydrazine sulfate concentration was necessary.
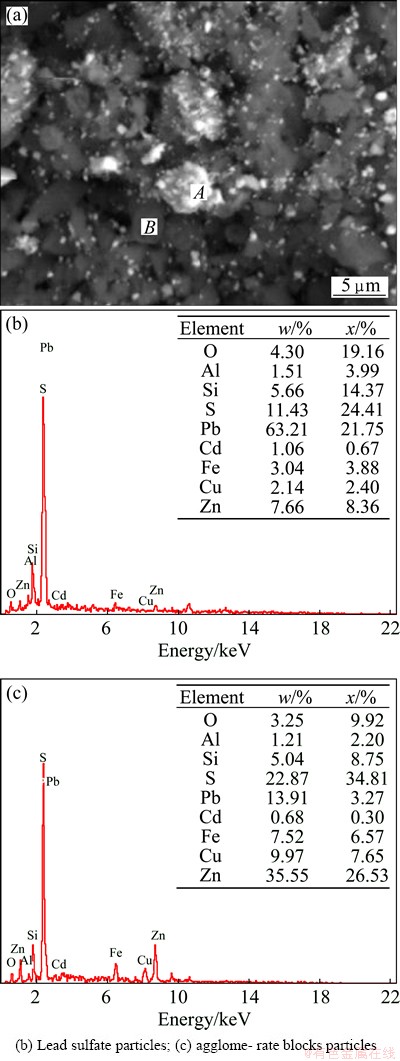
Fig. 12 SEM image (a) and EDS patterns (b,c) of ZNLR particles after leaching
References
[1] MIN Xiao-bo, XIE Xian-de, CHAI Li-yuan, LIANG Yan-jie, LI Mi, KE Yong. Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(1): 208-218.
[2] SADEGH SAFARZADEH M, BAFGHI M S, MORADKHANI D, OJAGHI ILKHCHI M. A review on hydrometallurgical extraction and recovery of cadmium from various resources [J]. Minerals Engineering, 2007, 20(3): 211-220.
[3] JHA M K, KUMAR V, JEONG J, LEE J C. Review on solvent extraction of cadmium from various solutions [J]. Hydrometallurgy, 2012, 111-112: 1-9.
[4] BI Xiang-yang, FENG Xin-bin, YANG Yuan-gen, QIU Guang-le, LI Guang-hui. Quantitative assessment of cadmium emission from zinc smelting and its influences on the surface soils and mosses in hezhang county, southwestern China [J]. Atmospheric Environment, 2006, 40(22): 4228-4233.
[5] GRAYDON J W, KIRK D W. The mechanism of ferrite formation from iron sulfides during zinc roasting [J]. Metallurgical Transactions B, 1988, 19(5): 777-785.
[6] BALARINI J C, POLLI L D O, MIRANDA T L S, CASTRO R M Z D, SALUM A. Importance of roasted sulphide concentrates characterization in the hydrometallurgical extraction of zinc [J]. Minerals Engineering, 2008, 21(1): 100-110.
[7] ZHANG Yan-juan, LI Xuan-hai, PAN Liu-ping, WEI Yan-song, LIANG Xin-yuan. Studies on the kinetics of zinc and indium extraction from indium-bearing zinc ferrite [J]. Hydrometallurgy, 2010, 100(3-4): 172-176.
[8] LI Xuan-hai, ZHANG Yan-juan, PAN Liu-ping, LIANG Xin-yuan, LI Xue-ping. Effect of mechanical activation on dissolution kinetics of neutral leach residue of zinc calcine in sulphuric acid [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1512-1519.
[9] TURAN M D,  Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1-4): 169-176.
Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1-4): 169-176.
[10] CHI K H, CHANG M B, CHANG S H. Measurement of atmospheric PCDD/F and PCB distributions in the vicinity area of Waelz plant during different operating stages [J]. Science of the Total Environment, 2008, 391(1): 114-123.
[11] HUNG P C, CHI K H, CHEN M L, CHANG M B. Characteristics of dioxin emissions from a Waelz plant with acid and basic kiln mode [J]. Journal of hazardous materials, 2012, 201-202: 229-235.
[12]  ATA N O. Optimization of dissolution of metals from Waelz sintering waste (WSW) by hydrochloric acid solutions [J]. Chemical Engineering Journal, 2010, 162(2): 718-722.
ATA N O. Optimization of dissolution of metals from Waelz sintering waste (WSW) by hydrochloric acid solutions [J]. Chemical Engineering Journal, 2010, 162(2): 718-722.
[13] ZHANG Ya-li, YU Xian-jin, LI Xiao-bin. Zinc recovery from franklinite by sulphation roasting [J]. Hydrometallurgy, 2011, 109(3-4): 211-214.
[14] LECLERC N, MEUX E, LECUIRE J M. Hydrometallurgical extraction of zinc from zinc ferrites [J]. Hydrometallurgy, 2003, 70(1-3): 175-183.
[15] LANGOV  MAT SEK D. Zinc recovery from steel-making wastes by acid pressure leaching and hematite precipitation [J]. Hydrometallurgy, 2010, 101(3): 171-173.
MAT SEK D. Zinc recovery from steel-making wastes by acid pressure leaching and hematite precipitation [J]. Hydrometallurgy, 2010, 101(3): 171-173.
[16] LANGOV  MAT SEK D. Selective leaching of zinc from zinc ferrite with hydrochloric acid [J]. Hydrometallurgy, 2009, 95(3-4): 179-182.
MAT SEK D. Selective leaching of zinc from zinc ferrite with hydrochloric acid [J]. Hydrometallurgy, 2009, 95(3-4): 179-182.
[17] KIM T H, SENANAYAKE G, KANG J G, SOHN JS, RHEE K I, LEE S W, SHIN S M. Reductive acid leaching of spent zinc–carbon batteries and oxidative precipitation of Mn-Zn ferrite nanoparticles [J]. Hydrometallurgy, 2009, 96(1-2): 154-158.
[18] DAS G K, de LANGE J A B. Reductive atmospheric acid leaching of West Australian smectitic nickel laterite in the presence of sulphur dioxide and copper(II) [J]. Hydrometallurgy, 2011, 105(3-4): 264-269.
[19] SUN Wei-yi, SU Shi-jun, WANG Qing-yuan, DING Sang-lan. Lab-scale circulation process of electrolytic manganese production with low-grade pyrolusite leaching by SO2 [J]. Hydrometallurgy, 2013, 133(0): 118-125.
[20] SENANAYAKE G, CHILDS J, AKERSTROM B D, PUGAEV D. Reductive acid leaching of laterite and metal oxides—A review with new data for Fe(Ni,Co)OOH and a limonitic ore [J]. Hydrometallurgy, 2011, 110(1-4): 13-32.
[21] NAYL A A, ISMAIL I M, ALY H F. Recovery of pure MnSO4·H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide [J]. International Journal of Mineral Processing, 2011, 100(3-4): 116-123.
[22] DUTRA A J B, PAIVA P R P, TAVARES L M. Alkaline leaching of zinc from electric arc furnace steel dust [J]. Minerals Engineering, 2006, 19(5): 478-485.
[23] LI Mi, PENG Bing, CHAI Li-yuan, PENG Ning, XIE Xian-de, YAN Huan. Technological mineralogy and environmental activity of zinc leaching residue from zinc hydrometallurgical process [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1480-1488.
[24] LANGOV  RIPLOV J, VALLOV S. Atmospheric leaching of steel-making wastes and the precipitation of goethite from the ferric sulphate solution [J]. Hydrometallurgy, 2007, 87(3-4): 157-162.
RIPLOV J, VALLOV S. Atmospheric leaching of steel-making wastes and the precipitation of goethite from the ferric sulphate solution [J]. Hydrometallurgy, 2007, 87(3-4): 157-162.
[25] FILIPPOU D, DEMOPOULOS G. Steady-state modeling of zinc-ferrite hot-acid leaching [J]. Metall and Materi Trans B, 1997, 28(4): 701-711.
[26] RAMACHANDRA SARMA V, DEO K, BISWAS A. Dissolution of zinc ferrite samples in acids [J]. Hydrometallurgy, 1976, 2(2): 171-184.
[27] FILIPPOU D, DEMOPOULOS G P. A reaction kinetic model for the leaching of industrial zinc ferrite particulates in sulphuric acid media [J]. Canadian Metallurgical Quarterly, 1992, 31(1): 41-54.
硫酸肼还原浸出锌中浸渣中镉
张 纯1,2,闵小波1,3,张建强1,王 密1,周波生1,沈 忱1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 湖南城市学院,市政与测绘工程学院,益阳 413000;
3. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083
摘 要:湿法炼锌中性浸出渣(中浸渣)是含有Cd和Zn等重金属元素的一种危险中间物料,对环境造成严重危害。本研究所用锌中浸渣含有约35.99% Zn、15.93% Fe和0.26% Cd,而Cd主要以铁酸盐的形式存在。研究硫酸肼浓度、硫酸初始浓度、温度、时间以及液固比对酸性还原浸出锌中浸渣Cd、Zn和Fe浸出率的影响。结果表明,中浸渣在硫酸肼浓度为33.3 g/L、硫酸初始浓度为80 g/L、浸出温度为95 °C、液固比为10 mL/g、搅拌速度为400 r/min条件下还原浸出120 min,Cd、Zn和Fe的浸出率分别达90.81%、95.83%和94.19%。X射线衍射及扫描电镜-能谱分析显示还原浸出渣的主要物相为硫酸铅(PbSO4)以及硫酸锌肼复盐((N2H5)2Zn(SO4)2)。
关键词:还原酸浸;锌中浸渣;硫酸肼;镉
(Edited by Yun-bin HE)
Foundation item: Project (2012FJ1010) supported by the Key Project of Science and Technology of Hunan Province, China; Project (51474247) supported by the National Natural Science Foundation of China; Project (2012GS430201) supported by the Science and Technology Program for Public Wellbeing, China
Corresponding author: Xiao-bo MIN; Tel: +86-731-88830577; Fax: +86-731-88710171; E-mail: mxbcsu@163.com
DOI: 10.1016/S1003-6326(15)64068-7
Abstract: Zinc neutral leaching residue (ZNLR) from hydrometallurgical zinc smelting processing can be determined as hazardous intermediate containing considerable amounts of Cd and Zn which have great threats to the environment. The ZNLR contained approximately 35.99% Zn, 15.93% Fe and 0.26% Cd, and Cd mainly existed as ferrites in the ZNLR in this research. Reductive acid leaching of ZNLR was investigated. The effects of hydrazine sulfate concentration, initial sulfuric acid concentration, temperature, duration and liquid-to-solid ratio on the extraction of Cd, Zn and Fe were examined. The extraction efficiencies of Cd, Zn and Fe reached 90.81%, 95.83% and 94.19%, respectively when the leaching parameters were fixed as follows: hydrazine sulfate concentration, 33.3 g/L; sulfuric acid concentration, 80 g/L; temperature, 95 °C; duration of leaching, 120 min; liquid-to-solid ratio, 10 mL/g and agitation, 400 r/min. XRD and SEM-EDS analyses of the leaching residue confirmed that lead sulfate (PbSO4) and hydrazinium zinc sulfate((N2H5)2Zn(SO4)2) were the main phases remaining in the reductive leaching residue.


