
Fabrication and wear properties of co-deposited Ni-Cr nanocomposite coatings
ZHOU Yue-bo(周月波), ZHAO Guo-gang(赵国刚), ZHANG Hai-jun(张海军)
College of Materials Science and Engineering, Heilongjiang Institute of Science and Technology,Harbin 150027, China
Received 13 October 2008; accepted 6 March 2009
Abstract:
Ni-Cr nanocomposite coatings with different Cr particles contents were developed by electrodeposition method from a nickel sulfate solution containing different concentrations of Cr nanoparticle with an average particle size of 40 nm. The characteristics of the coatings were assessed by scanning electron microscopy and microhardness test. The friction and wear performances of Ni-Cr nanocomposite coatings and pure Ni film were comparatively investigated, with the effect of the Cr content on the friction and wear behaviors to be emphasized. The results indicate the microhardness, friction and wear behaviors of Ni-Cr nanocomposite coatings are closely related with Cr particles content. The Ni-Cr nanocomposite coating with a lower Cr content of 4.0% shows somewhat increased microhardness and wear resistance than the pure Ni coating, while the Ni-Cr nanocomposite coating with a higher Cr content has much better wear resistance than the pure Ni coating. The effect of Cr nanoparticles on the microhardness and wear resistance was discussed.
Key words:
electrodeposition; nanocomposite coating; microhardness; wear;
1 Introduction
The composite electrodeposition technique is a low-cost and low-temperature method suitable for producing metal matrix composite coatings for diverse purpose such as enhancing wear and abrasion resistance. These coatings typically contain oxide particles or carbide particles in micrometer, such as Al2O3[1], La2O3[2] and SiC[3-4], in an electrodeposited matrix such as nickel. It has been proposed that electrodeposited metal Ni matrix-metal Cr particle composite coatings can be fabricated by codeposition of Ni and micrometer-sized Cr particles[5]. However, their wear behaviour has been less reported. ZHANG et al [6-10] developed electrodeposited Ni matrix/Cr nanoparticles composite coatings by co-deposition of Ni with Cr nanoparticles. The deposited Ni-Cr nanocomposite coatings exhibit a superior hot corrosion and oxidation resistance due to the formation of a continuous chromia scale[6-8], which can be used in power plants[8] operating on a wide range of fuels including natural gas, kerosene, diesel oils, residual oils, and gaseous fuels made from coal, biomass, and waste, etc. In these environments, erosion from solid particles and gas flow is one of the important factors leading to the recession of steel boiler components. Thus, a higher wear resistance is also needed for the as-codeposited Ni-Cr nanocomposite coatings used in power plants. However, there were less reports about the wear resistance of Ni-Cr nanocomposite coatings. In the present work, Ni matrix composite coatings containing nano-sized Cr particles were prepared and the friction and wear performances were analyzed. For comparison, the preparation and wear performance of pure Ni film were also carried out under the same conditions. The effect of content of codeposited Cr nanoparticles on the wear resistance of the nanocomposite coating was discussed.
2 Experimental
Pure nickel specimens with the size of 15 mm× 10 mm×2 mm were cut from a pure electrolytic nickel plate (99.9%) and then were abraded by 800# grit SiC waterproof paper. After being ultrasonically cleaned in acetone, they were electrodeposited (on all sides) with a 60 mm-thick film of Ni-Cr nanocomposite from a nickel sulfate bath containing 150 g/L NiSO4·7H2O, 15 g/L NH4Cl, 15 g/L H3BO3, 0.1 g/L C12H25NaSO4, and certain content of pure Cr nanoparticles (in spherical shape) with a average particle size of 40 nm. The suspensions were stirred for 24 h before deposition. Before the electrodeposition, the samples were degreased in alkaline solution, dipped in acid (10% HCl) and finally washed with distilled water. Magnetic stirring was employed at the cell bottom to maintain the uniform particles concentration and prevent sedimentation. The conditions were the current density of 3 A/dm2, the temperature of 35 ℃, pH 5.5-6.0, and the stirring rate of 600 r/min. For comparison, specimen of nickel with a 60 mm-thick Ni film was also deposited using the same parameters and the same bath but without adding Cr nanoparticles. After the deposition, the as-deposited samples were rinsed by using distilled water and then ultrasonically cleaned for analysis.
Surface morphologies and the compositions of Ni-Cr and pure Ni coatings were examined by a scanning electron microscope with energy dispersive X-ray analyzer (SEM/EDAX). The grain size of Ni matrix of the films was determined using the Scherrer’s equation[11]. Measurements of the Vickers microhardness were performed on the surface by using a microhardness tester under a load of 0.49 N for 10 s and the corresponding final values were determined as the average of 10 measurements.
The friction and wear tests were performed at room temperature on a ball-on-disc type tribometer with a constant rotation speed of 200 r/min, a constant radius of 2.5 mm and a load of 150 N under non-lubricated conditions. Si3N4 ceramic balls with 2 mm in diameter were used as the counter body. Each wear test lasted for 1 h for a total distance of 188.4 m. The friction coefficient and time were recorded automatically during the test. The mass loss of the samples, to an accuracy of 0.01 mg, was detected to evaluate the wear loss of the coatings. Three replicate wear tests were carried out so as to minimize data scattering, and every value reported was an average of three measurements. After wear test, the worn surfaces were analyzed using SEM/EDAX.
3 Results and discussion
3.1 Coating microstructure
Fig.1 shows the relationship between the content (mass fraction) of codeposited Cr and the concentration of Cr particles in the bath at 3 A/dm2, 35 ℃ and 600 r/min. It is seen that, the content of the codeposited Cr particles in the composite coatings increases with an increase of Cr concentration in the plating bath, which is in agreement with result in Ref.[7]. The highest content of codeposited Cr particles is achieved at Cr concentration of 100 g/L. The curve is quite similar to the well-known Langmuir adsorption isotherms, supporting a mechanism based on an adsorption effect. The codeposition of Cr by the electrodeposition technique may be attributed to the adsorption of Cr particles on the cathode surface, as suggested by the two-step adsorption model[12]. Once the particle is adsorbed, metal begins building around the cathode slowly, encapsulating and incorporating the particles. The highest concentration of Cr particles on the codeposit is due to saturation in adsorption on cathode surface.
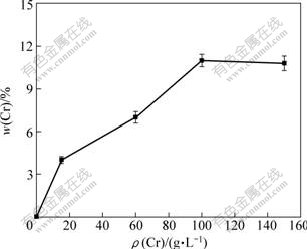
Fig.1 Effect of Cr concentration in bath on content of Cr in coatings
SEM surface morphologies of the pure Ni and Ni-Cr nanocomposite coatings containing different contents of Cr particles are shown in Fig.2. The pure Ni deposit shows a regular pyramidal structure. Whereas, with the codeposition of Cr nanoparticles (as indicated by the arrow in Fig.2(b)), the morphology is changed to nodular microstructure, as seen in Figs.2(b)-(d). It is evident that Cr nanoparticles are uniformly distributed in the Ni matrix. Meanwhile, there are more Cr nanoparticles on the surface because of the increased concentration of Cr nanoparticles in the bath, which is consistent with the EDAX results.
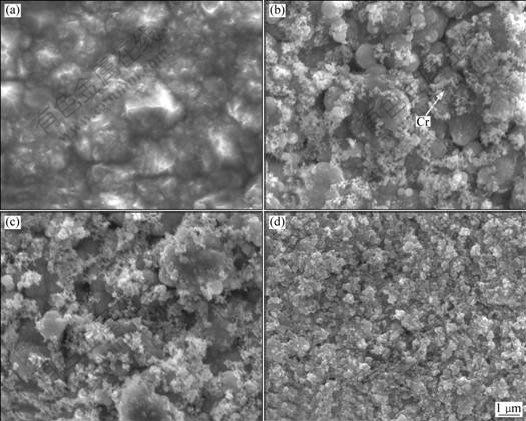
Fig.2 SEM images of surface morphologies of pure Ni and Ni-Cr nanocomposite coatings containing different contents of Cr nanoparticles: (a) Pure Ni; (b) 4.0% Cr; (c) 7.0% Cr; (d) 11.0% Cr
The crystallinity and phase characterization of pure Ni and Ni-11%Cr nanocomposite coating were carried out using XRD. During the processing of the pure Ni coating from the particle-free bath, both Ni deposits grow preferentially on the Ni {200} orientated planes. However, such a preferred growth direction is suppressed by the incorporation of Cr nanoparticles into the Ni matrix, which leads to a homogeneous growth of Ni deposits on {111}, {200}, and {220} planes, as seen in Fig.3. In the meantime, the grain size of Ni deposits becomes somewhat small. For example, it is around 60 nm in average for the pure Ni film, and 52 nm for the Ni-11.0%Cr nanocomposite coating. The reason is understandable that the nanoparticles have a high surface energy. Once being included into the cathode surface during electrodeposition, they would promote the nucleation of fresh Ni deposits rather than the growth of the existing Ni deposits. The promotion of the deposits nucleation favors the grain refinement. Similar results have been reported elsewhere[6-10].
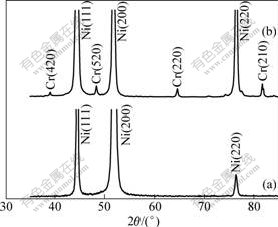
Fig.3 XRD patterns of as-deposited pure Ni film (a) and Ni-11.0%Cr nanocomposite coating (b)
3.2 Wear resistance
Fig.4 shows the friction coefficient of the as-deposited Ni film and Ni-Cr nanocomposites with various Cr contents under non-lubricated conditions at load of 150 N. It is found that, during the first 1 200 cycles, all coatings exhibit a very low friction coefficient of 0.15-0.20. For the Ni film, it increases dramatically to 1.0 after 6 000 cycles and then maintains at a constant level. For Ni-4.0%Cr nanocomposite coating, it increases to 0.65 below 7 500 cycles and increases dramatically to 1.0 after 8 500 cycles and then maintains at a constant level. For Ni-7.0%Cr nanocomposite coating, it exhibits little change during the first 4 000 cycles, and increases to 0.8 after 10 000 cycles. However, for Ni-11%Cr nano- composite, it exhibits little change and keeps stable during the test. It is found that, with the increase of Cr nanoparticles content, the curves descend and become stable gradually. The curve for Ni-11%Cr nanocomposite coating is very flat and the fluctuation becomes lower than that of other curves. From Fig.4 it can be seen that the friction coefficient of the Ni-Cr nanocomposite coatings decreases gradually with increasing the content of Cr nanoparticles in the coatings.
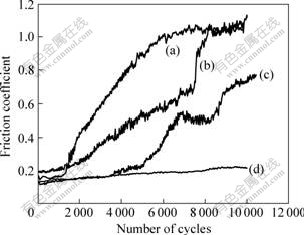
Fig.4 Typical friction coefficients of pure Ni and Ni-Cr nanocomposite coating containing different contents of Cr nanoparticles: (a) Pure Ni; (b) 4.0% Cr; (c) 7.0% Cr; (d) 11.0% Cr
The microhardness and wear mass loss of pure Ni film and Ni-Cr nanocomposites coatings as a function of the mass fraction of the codeposited Cr nanoparticles are shown in Fig.5. The pure Ni coating has a minimum microhardness and maximum wear mass loss. With the increase of Cr nanoparticles content in the Ni matrix, the microhardness increases, while the wear mass loss reduces. The results from Fig.4 and Fig.5 strongly suggest that the friction and wear behaviors of Ni-Cr nanocomposite coatings are closely related with Cr content. The composite coating with a lower Cr content of 4.0% shows somewhat increased wear resistance than the pure Ni film, while the composite coating with a higher Cr content of 11.0% has much better wear resistance than the pure Ni coating. This implies that the friction and wear behaviors of the Ni-Cr nanocomposite coatings can be largely improved by increasing the Cr content in the deposit. The wear resistance and hardness of the electrodeposited composite coatings are found to be related to each other as well as to the particle content in the coating. Coatings with the maximum hardness exhibit the highest wear resistance. In the experiment, the Ni-11.0%Cr nanocomposite coating is recorded with the lowest friction coefficient and the minimum wear loss, therefore exhibits excellent tribological performance.
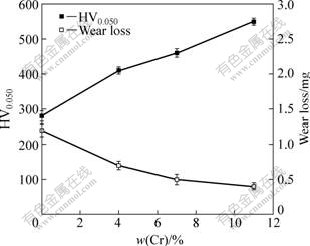
Fig.5 Effect of content of codeposited Cr nanoparticles on microhardness and wear mass loss of Ni-Cr nanocomposite coatings
The difference in the wear behavior of the as-deposited Ni film and Ni-Cr nanocomposite coatings containing different contents of Cr nanoparticles under non-lubricated conditions can be further verified by the worn surface morphologies shown in Fig.6. For the pure Ni coating, cracking and spalling can be observed on the worn surface, as shown in Fig.6(a). The presence of the cracking and spalling yields bigger worn debris. This reveals that, without the incorporation of Cr nanoparticles in Ni deposit, wear resistance of the pure Ni coating is rather weak. It is seen in Fig.6(b) that there is still spalling trace with less width and depth on the worn surface of the Ni-4.0%Cr nanocomposite coating. Furthermore, increasing the content of codeposited Cr nanoparticles, some obvious abrasive grooves are noticed on the worn surface of the Ni-7.0%Cr nanocomposite coating, as shown in Fig.6(c). For Ni-11.0%Cr nanocomposite coating, the abrasive grooves on the worn surface are significantly decreased, and the feature shows less abrasive width and depth, as seen in Fig.6(d). Fig.6(e) shows the higher magnification back scattering image of Fig.6(d). EDAX analysis reveals the black or gray spot area in Fig.6(e) with higher Cr content than the other area, suggesting that they were the deposited Cr nanoparticles. From Fig.6(e), it can be found that the dark Cr nanoparticles are, in general, homogeneously dispersed in the Ni-Cr nanocomposite coating, although some of them form agglomerated clusters. By comparison of the worn surfaces of two coatings in Fig.6(a) and Fig.6(d), it can be found that the Ni-Cr nanocomposite coatings exhibit less abrasive width and depth. It is suggested that, the incorporation of Cr particles in the matrix can largely reduce the wear of the nickel composite coatings; furthermore, the wear resistance of the Ni-Cr nanocomposite coating is enhanced with increasing Cr nanoparticles in the composite coatings.
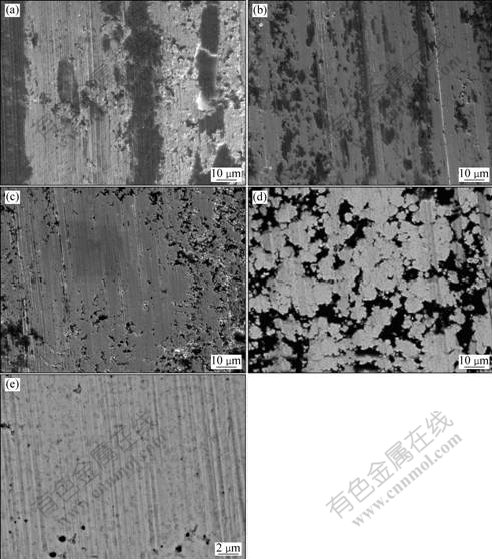
Fig.6 SEM image of worn surface of pure Ni and Ni-Cr nanocomposite coating containing different contents of Cr nanoparticles: (a) Pure Ni; (b) 4.0% Cr; (c) 7.0% Cr; (d) 11.0%Cr, in lower magnification; (e) 11.0%Cr, in higher magnification
The above wear results suggest that with the increase of Cr nanoparticles content in the Ni matrix, the microhardness increases, while the wear mass loss reduces. ARCHARD[13] calculated the wear rates of all coatings using the equation of ?V=kLS/H, where ?V is the wear volume; L is the normal load; S is the sliding distance every cycle; H is the microhardness; and k is the coefficient of wear loss. In the experiment, k,L and S were constant values. Thus, there is a linear relationship between ?V and 1/H. However, there is a linear relationship between the volume wear loss ?V and the wear mass loss, thus, there also exists a linear relationship between the wear mass loss and 1/H, as seen in Fig.7. There are three reasons for the increase in hardness[14]: particle-strengthening, dispersion- strengthening and grain refining. A particle- strengthening composite contains more than 20% (volume fraction) of particles larger than 1 mm. The load is carried out by both the matrix and the particles. Strengthening is achieved because particles restrain matrix deformation by a mechanical constraint. Dispersion-strengthening is associated with the incorporation of fine particles with a particle diameter ranging from 0.01 to 1 μm and volume fraction lower than 15%. In this case, the matrix carries the load and the small particles hinder dislocation motion[15-19]. The third mechanism is related to the nucleation of small grains on the surface of the incorporated particles, resulting in a general structural refinement. In this case, the presence of smaller grains impedes dislocation motion, resulting in an increase in microhardness. Thus, for the Ni-Cr nanocomposite coatings in this experiment, the enhancement in the hardness is related to the dispersion-strengthening effect caused by Cr nanoparticles in the composite coatings, which impedes the motion of dislocations in metallic matrix. FENG et al[20] reported that embedding of nanoparticles perturbs the crystal growth of Ni, inducing a reduction in the crystal size and giving deposits with significantly increased hardness values. QU et al[21] also reported that the existence of nano-sized CeO2 particles, as the second phase, reduces the grain size of Ni-matrix. Accordingly, the higher microhardness value and wear resistance of the Ni-Cr nanocomposite coatings may be also due to the decrease of the grain size of Ni-matrix of the composites, which is favored by the nano-sized Cr particles. With the grain refinement of Ni matrix, the load carrying ability and the resistance for plastic deformation[9, 15-17, 20-21] increase. At the same time, the codeposited Cr nanoparticles gradually protrude out of the matrix during the friction process, which carries the loads transferred from the matrix, and as a result, the amount of thermal plastic deformation and scuffing wear at high temperature caused by the heat generated in the sliding is reduced. These are the reasons why the co-deposited Ni-Cr nanocomposites with high Cr particles content exhibit lower friction coefficient and better wear resistance than the as-deposited pure Ni film.

Fig.7 Liner relationship between microhardness and wear mass loss of Ni-Cr nanocomposite coatings
4 Conclusions
1) The content of Cr particles in Ni-Cr nanocomposite coatings increases with increasing concentration of Cr particles in the plating bath.
2) The microhardness, friction and wear behaviors of Ni-Cr nanocomposite coatings are closely related with Cr particles content. The Ni-Cr nanocomposite coating with a lower Cr content of 4.0% shows somewhat increased microhardness and wear resistance than the pure Ni coating, while the Ni-Cr nanocomposite coating with a higher Cr content has much better wear resistance than the pure Ni coating. Namely, the microhardness and wear resistance increase with the increase of Cr nanoparticles content in the Ni matrix, which can be attributed to the fact that the incorporation of nanometer Cr particles in the Ni matrix greatly increases the hardness of the composite coating through the dispersion-strengthening effect of the codeposited Cr nanoparticles.
References
[1] FENG Qiu-yuan,LI Ting-ju, YUE Hong-yuan, BAI Fu-dong, JIN Jun-ze. Preparation and characterization of nickel nano-Al2O3 composite coatings by sediment codeposition [J]. Applied Surface Science, 2008, 254: 2262-2268.
[2] XUE Yu-jun, ZHU Di, ZHAO Fei. Electrodeposition and mechanical properties of Ni–La2O3 nanocomposites [J]. Journal of Materials Science, 2004, 39: 4063-4066.
[3] AAL A A, KHALED I M, HAMID Z A. Enhancement of wear resistance of ductile cast iron by Ni–SiC composite coating [J]. Wear, 2006, 260: 1070-1075.
[4] LEE H K, LEE H Y, JEON J M. Codeposition of micro- and nano-sized SiC particles in the nickel matrix composite coatings obtained by electroplating [J]. Sur Coat Technol, 2007, 201: 4711-4717.
[5] BAZZARD R, BODEN P J. Nickel-chromium alloys by codeposition (Part Ⅰ) : Codeposition of chromium particles in a nickel matrix [J]. Trans Inst Met Finishing, 1972, 50: 63-69.
[6] ZHANG Y, PENG X, WANG F. Development and oxidation at 800 ℃ of a novel electrodeposited Ni-Cr nanocomposite film [J]. Materials Letters, 2004, 58: 1134-1138.
[7] PENG X, ZHOU Y, ZHANG Y, WANG F. On the development and the oxidation of novel Ni-Cr and Ni-Al nanocoatings by composite electrodeposition [J]. Mater Sci Forum, 2004, 461/464: 409-416.
[8] ZHANG C, PENG X, ZHAO J, WANG F. Hot corrosion of an electrodeposited Ni-11% Cr nanocomposite under molten Na2SO4-K2SO4-NaCl [J]. Journal of the Electrochemical Society, 2005, 152(9): B321-B326.
[9] PENG X, ZHANG Y, WANG F. A novel electrodeposited Ni-Cr nanocomposite with increased resistance to pitting corrosion in 3.5% NaCl solution [J]. Electrochemical and Solid-state Letters, 2005, 8(9): B46-B49.
[10] PENG X, ZHANG Y, ZHAO J, WANG F. Electrochemical corrosion performance in 3.5% NaCl of the electrodeposited nanocrystalline Ni films with and without dispersions of Cr nanoparticles [J]. Electrochimica Acta, 2006, 51: 4922-4927.
[11] GULLITY B D. Elements of X-ray diffraction[M]. 2nd ed. New York: Addison-Wesley Publishing House, 1978: 102-106.
[12] GUGLIELMI N. Kinetics of the deposition of inert particles from electrolytic baths[J]. J Electrochem Soc, 1972, 119: 1009-1012.
[13] ARCHARD J F. Contact and rubbing of flat surface [J]. J Appl Phys, 1953, 24: 981-988.
[14] FRATARI R Q, ROBIN A. Production and characterization of electrolytic nickel–niobium composite coatings [J]. Sur Coating Technol, 2006, 200: 4082-4090.
[15] GYFTOU P, STROUMBOULI M, PAVLATOU E A, ASIMIDIS P, SPYRELLIS N. Tribological study of Ni matrix composite coatings containing nano and micro SiC particles [J]. Electrochimica Acta, 2005, 50: 4544-4550
[16] XUE Yu-jun, ZHU Di, ZHAO Fei. Electrodeposition and mechanical properties of Ni-La2O3 nanocomposites[J]. Journal of Materials Science, 2004, 39: 4063-4066.
[17] XUE Yu-jun, JIA Xian-zhao, ZHOU Yan-wei, MA Wei, LI Ji-shun. Tribological performance of Ni-CeO2 composite coatings by electrodeposition [J]. Surf Coat Technol, 2005, 200: 5677-5687.
[18] GARCIA L, FRANSAER J, CELIS J P. Electrodeposition and sliding wear resistance of nickel composite coating containing micro and submicron SiC particles [J]. Sur Coat Technol, 2001, 148: 171-178.
[19] WU G, LI N, ZHOU D R, MITSUO K. Electrodeposited Co-Ni-Al2O3 composite coatings [J]. Surf Coat Technol, 2004, 176: 157-164.
[20] FENG Q Y, LI T G, YUE H Y, QI K, BAI F D, LIN J Z. Preparation and characterization of nickel nano-Al2O3 composite coatings by sediment co-deposition [J]. Applied Surface Science,2008,254: 2262-2268.
[21] QU N S, ZHU D, CHAN K C. Fabrication of Ni-CeO2 nanocomposite by electrodeposition [J]. Scripta Materialia, 2006, 54: 1421-1425.
Foundation item: Project(11531319) supported by Scientific Research Fund of Heilongjiang Provincial Education Department, China; Project(9951Z012) supported by the Major Programs of the Heilongjiang Provincial Education Department, China; Project(06-13) supported by the Scientific Research Startup Foundation of Heilongjiang Institute of Science and Technology, China
Corresponding author: ZHOU Yue-bo; Tel: +86-451-88036526: E-mail: zhouyuebo760309@163.com; ybzhou@imr.ac.cn
DOI: 10.1016/S1003-6326(09)60104-7
(Edited by YANG Hua)


