J. Cent. South Univ. (2016) 23: 2513-2519
DOI: 10.1007/s11771-016-3311-x
Application and characterization of grafted polytetrafluoroethylene fiber for enhanced adsorption of Cu(II) in aqueous solutions
LI Jiong-hui(厉炯慧)1, MIAO Xi-xi(缪喜喜)2, CHEN Xin-yi(陈昕怡)2, LU Li(逯丽)2,
YANG Yi(杨奕)2, FU Ya-qin(傅雅琴)3, XIONG Chun-hua(熊春华)4
1. School of Environmental Science and Engineering, Zhejiang Gongshang University, Hangzhou 310012, China;
2. Department of Applied Chemistry, Zhejiang Gongshang University, Hangzhou 310012, China;
3. School of Materials and Textile, Zhejiang Sci-Tech University, Hangzhou 310018, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
An enhanced adsorption and desorption procedure of Cu(II) onto green synthesized acrylic acid grafted polytetrafluoroethylene fiber (i.e. AA-PTFE) was conducted with various chemical methods. The results show that the optimal adsorption condition is in acetic acid, sodium acetate (HAc-NaAc) buffer solution (pH=6.80) with the initial concentration of 0.2 mg/mL. The process is very fast initially and equilibrium time is 12 h with a high Cu(II) uptake of 112.26 mg/g at 298 K. Various thermodynamic parameters indicate that the adsorption process is spontaneous and endothermic in nature. In the elution test, 2 mol/L HCl solution achieves satisfactory elution rate and shows no significant decrease after 5 adsorption-desorption cycle, which indicates that AA-PTFE can be regenerated and reused, and due to which a reasonable amount of nondegradable polymer material is avoided in industrial use. Finally, PTFE, AA-PTFE fiber, and Cu(II) loaded AA-PTFE fiber were characterized with various techniques, including IR spectroscopic technique, SEM and EDS.
Key words:
PTFE; adsorption; Cu(II); SEM; EDS;
1 Introduction
In recent years, there has been a gradual accumulation of electrical and electronic product waste, including an enormous amount of metal recourses such as precious and heavy metals. Also, because of the value and scarcity of the precious metals, gold, silver, platinum and palladium, it has become even more economically imperative to recover them [1-3]. By the year 2020, the amount of waste electrical and electronic products in China will reach a rather considerable amount of 137 million tonnage and will continue to grow [4]. On regard of shortage of natural recourses and the environmental disruption we’ve already caused, it is really urgent to have those heavy metals recovered properly.
Many different techniques have been proposed for separation and preconcentration of trace metals, including precipitation, solvent extraction, membrane separation, reverse osmosis, electro dialysis and solid phase extraction (SPE) [5-11]. Yet many problems remain unsolved, such as the low durability and unsatisfactory selectivity of the electro dialysis membrane [12]. These techniques also have certain disadvantages, such as low speed of adsorption process, poor thermal stability and regeneration stability. For example, in a study by TUZEN and SOYLAK [13], the capacity of sorbent for metals was found lower than 10 mg/g, but our research achieved more than 100 mg/g metals from solutions.
Polytetrafluoroethylene (PTFE) material is widely used in fuel-cell industry as well as in many other fields [14-18]. Among all those researches, PTFE or grafted PTFE material were presented in the form of powder or membrane, which are both too difficult to separate and regenerate. Alternatively, the ion exchange fiber was chosen due to its fabulous adsorption behavior for rare earth metals (higher adsorption speed, sound regeneration ability and significant convenience) in our previous studies [19].
In the present work, we synthesized a grafted polytetrafluoroethylene ion exchange fiber AA-PTFE (acrylic acid grafted polytetrafluoroethylene fiber) with radiation and applied it in the following research for the first attempt. As a result, we got new functional groups containing C=O and —OH on the PTFE fiber, which has good application to the separation and removal of Cu(II) in aqueous solutions and excellent reusable property. Study of the adsorption of Cu(II) on AA-PTFE (acrylic acid grafted polytetrafluoroethylene fiber) is optimized in batch and column processes. Finally, IR spectroscopic technique, scanning electron microscopic (SEM) technique and energy dispersive spectroscopic (EDS) technique were adopted to characterize the polytetrafluoroethylene fiber (PTFE), AA-PTFE fiber and Cu(II) loaded AA-PTFE fiber (acrylic acid grafted polytetrafluoroethylene fiber). Above all, our work requires a multidisciplinary approach that involves not just materials science and chemical engineering, but also environmental science, using efficient biometrical methods (RSM), which is seldom applied in the field of chemical industry.
2 Materials and methods
2.1 Apparatus
The Cu(II) was determined with Shimadzu UV-2550 UV-VIS spectrophotometer. Mettler toledo delta 320 pH meter was used for measuring pH of solutions. Batch experiments were carried out in the DSHZ-300A temperature constant shaking machine. The water used in the present work was purified using Molresearch analysis-type ultra-pure water machine. IR spectra and scanning electron micrographs (SEM) for the samples were obtained from a Nicolet 380 FTIR spectroscopy and a HITACHI S-3000N scanning electron microscope, respectively. Dispersive spectroscopic analysis was performed on INCA ENERGY 350 dispersive spectrometer (Oxford, England).
2.2 Materials and reagents
PTFE fiber was purchased from Tongchuang Co., Beijing, China (Φ=20 μm). HAc-NaAc buffer solutions with pH 4.0-6.8 were prepared from the NaAc and HAc to control the pH of the solutions. The 0.5% solution of the chromophoric reagent xylenol orange was obtained by dissolving 0.5 g xylenol orange powder into 100 mL deionized water. All other chemicals were of analytical grade and purified water was used throughout. All other chemicals were of analytical grade and purified water was used throughout.
2.3 Preparation of ion exchange fiber
The PTFE fibers were sequentially washed with double distilled water (DDW), HCl solution, DDW, NaOH solution, DDW and acetone, and then dried in a vacuum oven at 323 K for 48 h. The 15 g dried PTFE fiber and 2.8% Mohr’s salt were added into a certain amount of acrylic acid (AAc) and deionized water in a 500 mL flask and sealed after the oxygen was removed completely by a high purity nitrogen flow, and then directly subjected to 60Co irradiation at a dose rate of 0.5 kGy/h for a given time. After irradiation, the grafted PTFE fibers were filtered and repeatedly washed with certain kind of solutions mentioned above for three times and dried in a vacuum oven at 323 K for 48 h and then weighed.
2.4 Experimental design for RSM study
Response surface methodology (RSM) was used to optimize the adsorption conditions of AA-PTFE fiber for Cu(II) from aqueous solutions. Factor combinations were obtained by the application of a Box-Behnken design (BBD) using software Design-Expert 8.0.5b. In this part, optimal process conditions with regard to pH value, ion concentration (0.1-0.3 mg/mL) and the amount of fibers (10-20 mg) were identified.
2.5 Adsorption experiments
The experiments were carried out in batch vessels and glass columns. In the batch experiments, a desired amount of dried AA-PTFE was weighed and added into a conical flask, in which a desired volume of buffer solution was added. After 24 h, a required amount of standard Cu(II) solution was put in. The flask was shaken in a shaker at constant temperature. The upper layer of clear solution was taken for analysis until adsorption equilibrium reached. Continuous flow adsorption experiments were conducted in a vertical glass column with Cu(II) ion solution fed from the top at a fixed flow rate. The Cu(II) solutions at the outlet of the column were collected periodically and analyzed for the Cu(II) concentration using a UV-visible spectro- photometer at 555 nm. The flow through the column was continued till the outlet and inlet concentrations were equal.
2.6 Analytical method
A solution containing less than 36 μg Cu(II) was accurately added into a 25 mL colorimetric tube, and then 1.0 mL of 0.5% xylenol orange solution and 10 mL pH 5.7 (CH2)6N4-HCl buffer solution were added as well. After adding deionized water to the mark of colorimetric tube, the absorbency was determined in a 1 cm colorimetric vessel at wavelength of 555 nm and compared with blank test. The adsorption capacity (Q) of Cu(II) on AA-PTFE was calculated with the following formula:
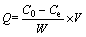 (1)
(1)
The distribution coefficient (D) of Cu(II) ions between the aqueous phase and the solid phase can be directly obtained using
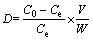 (2)
(2)
where C0 (mg/mL) and Ce (mg/mL) are the initial and equilibrium Cu(II) concentrations, respectively, and V/W is the ratio of the volume of metal solution (mL) to the amount of AA-PTFE (g) in a batch.
3 Results and discussion
3.1 Characterization of grafted polymerization with IR, SEM and EDS
After the AA-PTFE fibers were successfully synthesized with the optimal monomer concentration 37.5% and irradiation dose 24 kGy, the grafting degree was evaluated to be 47.0%. It is found that the characteristic vibration absorption peak of the bond C—OH in —COOH formed at 3405.19 cm-1. Bending vibration peak of the bond C—OH also was observed at 1445 cm-1. And the characteristic peak at 1720 cm-1 was formed by C=O in —COOH. IR spectroscopic technique was applied and indicated that acrylic acid and PTFE fiber were combined by forming chemical bonds rather than any kind of physical mixed compound (Fig. 1). And then, a scanning electron microscopy (SEM) equipped with an energy-dispersive X-ray spectroscopy (EDS) was used to characterize the morphologies and chemical compositions of the PTFE fiber and AA-PTFE fiber samples. Figures 2(a) and (b) show the SEM analysis results of PTFE and AA-PTFE accordingly. By comparing the surface of PTFE with that of grafted PTFE, it was observed that the smooth surface of the original PTFE turned into rougher after grafting, and oxygen atoms were observed due to the EDS results (Figs. 2(a) and (b)). This suggests that acrylic acid which contained O was grafted onto PTFE.
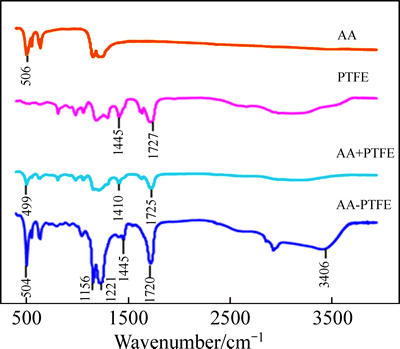
Fig. 1 FTIR spectra of AA, PTFE, AA together with PTFE and AA-PTFE
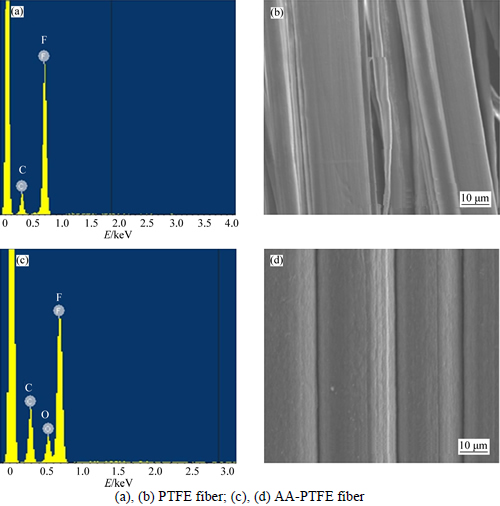
Fig. 2 EDS spectra and SEM images:
3.2 Batch studies
3.2.1 Adsorption conditions optimization by RSM
Box-Behnken design was used to develop a correlation between the adsorption conditions and adsorption capacity of AA-PTFE for Cu(II). A total of 17 runs for optimizing the three individual parameters at the BBD were used to determine the experimental error.
These types of plots (Fig. 3) show effects of two factors on the conversion rate and the other factor was kept at level zero. From the 3-D response surface plots, the optimal values of the parameters could be observed, and the interaction between each parameter can be easily understood. The results demonstrated that the combined treatment of 15 mg AA-PTFE fiber, a initial Cu(II) ion concentration of 0.2 mg/mL and a pH value of 6.8 was optimal for maximizing the adsorption capacity.
3.2.2 Adsorption isotherms
The adsorption data were analyzed to see whether the isotherm obeyed the Langmuir [20] and Freundlichm [21] isotherm models. The linear form of the Langmuir isotherms and Freundlich isotherm are represented by the following equations:
Langmuir isotherm: 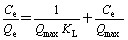 (3)
(3)
Freundlich isotherm: 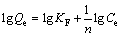 (4)
(4)
where Qe is the equilibrium Cu(II) ion concentration on the adsorbent (mg/g); Ce is the equilibrium Cu(II) ion concentration in solution (mg/mL); Qmax is the monolayer capacity of the adsorbent (mg/g) and KL is the Langmuir constant and related to the free energy of adsorption; KF is Freundlich constant and n (dimensionless) is the heterogeneity factor. The plots of Ce/Qe versus Ce (Langmuir) for the adsorption of Cu(II) ions onto AA-PTFE give a straight line of slope 1/Qmax and intercept 1/(QmaxKL); by plotting lgCe versus lgQe (Freundlich) to generate KF and n from the intercept and the slope, respectively. One of the Freundlich constants KF indicates the adsorption capacity of the adsorbent [22]. The other Freundlich constants n is a measure of the deviation from linearity of the adsorption. The numerical values of n at equilibrium lay between 1 and 10, indicating that Cu(II) ions is favorably adsorbed by AA-PTFE at all the studied temperatures.
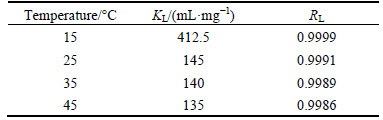
The Langmuir and Freundlich parameters for the adsorption of Cu(II) ions onto AA-PTFE is listed in Table 1. It is obvious from the data that the adsorption of Cu(II) ions onto AA-PTFE is fitted better to the Langmuir isotherm model than Freundlich isotherm model . The Langmuir model is the best-known isotherm for describing adsorption from aqueous solution. The Langmuir model supposes that the adsorption is localized in a monolayer.

Fig. 3 Influence of pH, fiber amount and ion concentration on adsorption of Cu(II) (T=298 K, 100 r/min):
3.2.3 Adsorption rate constant and kinetics study
The influence of contact time on the adsorption of Cu(II) ions onto AA-PTFE was investigated at various
Table 1 Isotherm constants for adsorption of Cu(II) on AA-PTFE at various temperatures
temperatures, i.e. 288, 298, 308 and 318 K. Results indicated that adsorption amount increased with increase of contact time. Furthermore, the loading half time t1/2 was 6 h and the maximum adsorption was observed after 12 h. Therefore, this interaction time could be regarded as equilibrium time.
Several adsorption kinetics curves were also obtained for Cu(II) on AA-PTFE. The kinetics of adsorption can be described by the liquid film diffusion model [23], using the Brykina method:
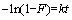 (5)
(5)
where F is the fractional attainment of equilibrium (F= Qt/Qe), and Qe and Qt are the amounts of Cu(II) ions adsorbed on the adsorbent at equilibrium and at various times, respectively; k is the adsorption rate constant. The experimental results were in accordance with equation and a straight line was obtained by plotting -ln(1-F) vs t. Therefore, the adsorption rate constant can be found from the slope of the straight line, and the correlation coefficient was obtained via linear fitting. The results are listed in Table 2. It can be concluded from the linear relationship of -ln(1-F) vs t that the liquid film spreading was the predominating step of the adsorption process [24-25].
Table 2 Liquid film diffusion model of AA-PTFE adsorption for Cu(II)

3.2.4 Elution tests and adsorption-desorption cycle
20.0 mg AA-PTFE was added into a mixed solution composed of pH 6.80 buffer solution and desired amount of Cu(II) solution. After equilibrium was reached, the concentration of Cu(II) in the aqueous phase was determined, and the adsorption capacity of the AA-PTFE for Cu(II) was obtained. Then, the AA-PTFE separated from aqueous phase was washed three times with pH 6.80 buffer solution. The AA-PTFE adsorbed Cu(II) was shaken with 30.0 mL HCl eluent. After equilibrium was reached, the concentration of Cu(II) in aqueous phase was determined and then the percentage of elution for Cu(II) was obtained. Finally, the 2 mol/L HCl solution was chose as an eluent.
It is considered that reusability is a very important factor for an effective absorption material. The “adsorption-desorption cycle” was also conducted by static experiment, which means we uploaded the AA- PTFE fiber with Cu(II) ions in a proper situation and then elute it under the primary condition we’ve figured out in the above study, and the adsorption-desorption cycle was repeated several times using the same material. The results (Table 3) clearly show that the AA-PTFE fiber could be used repeatedly without significantly losing its adsorption capacity.
Table 3 Adsorption-desorption cycle of Cu(II) onto AA-PTFE

3.3 Column studies
The performance of packed beds is described through the concept of the breakthrough curve. The breakthrough curve shows the loading behavior of Cu(II) to be removed from solution in a fixed bed and is usually expressed in terms of adsorbed Cu(II) concentration (Co-Ce) or normalized concentration defined as the ratio of effluent Cu(II) concentration to inlet Cu(II) concentration (Ce/Co) as a function of time or volume of effluent for a given bed height [26-28]. Total adsorbed Cu(II) quantity (Q, mg/g) in the column for a given feed concentration and flow rate is calculated as follows:
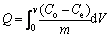 (6)
(6)
where m (g) is the mass of the adsorbent. The capacity value Q is obtained by graphical integration as 94.7 mg/g (Fig. 4).
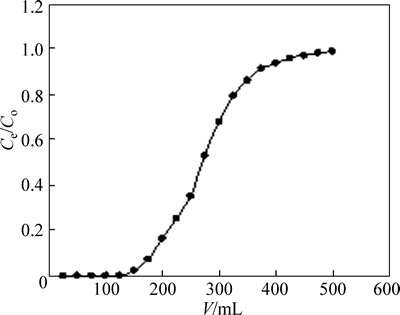
Fig. 4 Dynamic adsorption curve of Cu(II) ion onto AA-PTFE fibers (Fiber amount: 200 mg; C0=0.05 mg/mL; T=298 K; flow rate: 0.15 mL/min) With respect to the stripping of Cu(II) from AA-PTFE, the 2 mol/L HCl eluent was employed to conduct efficient elution in column at a certain flow rate. Result shows that the total volume of eluent was 50 mL and the desorption process took 2 h, after which further desorption was negligible. Therefore, the 2 mol/L HCl eluent could help in easy handling and removing of Cu(II).
3.4 Materials characterization
3.4.1 Infrared spectra of AA-PTFE
The spectra analysis of AA-PTFE, before and after Cu(II) is adsorbed, are conducted (Fig. 5). It is found that the characteristic peak of the bond C—OH shifts from 3405.19 cm-1 to 3427.17 cm-1. The characteristic peak of the bond C=O (1720.39 cm-1) weakened after being absorbed with Cu (II), and the new peak 1615.75 cm-1 formed. Therefore, the functional groups of AA-PTFE fiber, C—OH C=O, and Cu(II) are supposed to form chemical bonds.
3.4.2 SEM and EDS analysis of AA-PTFE
SEM and EDS were also used to characterize both the AA-PTFE fiber and those which had adsorbed Cu(II) ions. Figures 6(b) and (d) show the SEM analysis results of AA-PTFE before and after adsorption for Cu(II). By comparing the surface of AA-PTFE fiber with that of Cu(II)-loaded AA-PTFE fiber, it was observed that the smooth surface of the original AA-PTFE fiber turned into rough after adsorption, which was proven to be copper by further analysis with EDS (Figs. 6(a) and (c)). This suggests that Cu (II) ions were adsorbed onto AA-PTFE fiber.
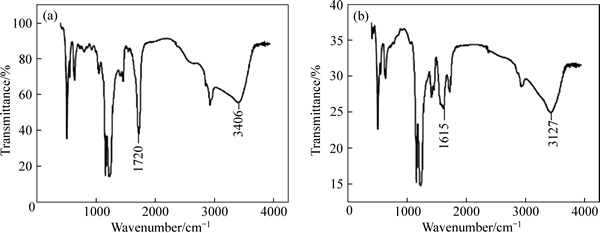
Fig. 5 FTIR spectra of AA-PTFE (a) and Cu(II)-loaded AA-PTFE (b)
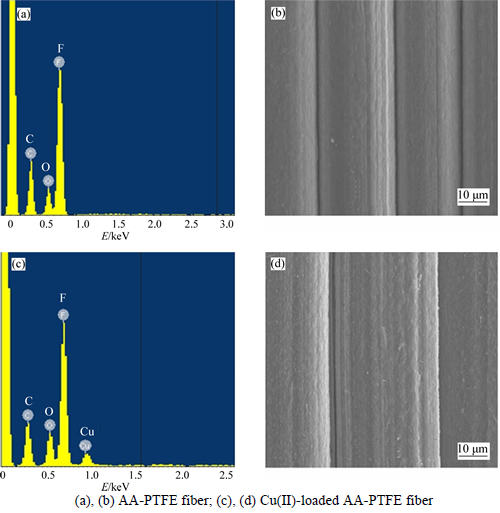
Fig. 6 EDS spectra and SEM images:
4 Conclusions
AA-PTFE fiber has a comparatively high sorption capacity, easy regeneration and efficient reusable ability towards Cu(II) in the HAc-NaAc system. Since rarely any heavy metal (Cu) removal studies by PTFE materials were reported, the experimental results may have a promising potential usage for understanding and optimizing the purification of Cu(II) containing wastewater and fit in with the environmental protection.
References
[1] Bratskaya S Y, Ustinov A Y, Azarova Y A, Pestov A V. Thiocarbamoyl chitosan: Synthesis, characterization and sorption of Au(III), Pt(IV), and Pd(II) [J].Journal of Polymer Science, 2011, 85: 854-861.
[2] Sopena L A S, Ruiz M, Pestov A V, Sastre A M, Yatluk Y, Guibal E. N-(2-(2-Pyridyl)ethyl)chitosan (PEC) for Pd(II) and Pt(IV) sorption from HCl solutions [J]. Cellulose, 2011, 18: 309-325.
[3] Won S W, Park J, Mao J, Yun Y S. Utilization of PEI-modified Corynebacterium glutamicum biomass for the recovery of Pd(II) in hydrochloric solution [J]. Bioresource Technology, 2011, 102: 3888-3893.
[4] WAGER P A, HISCHIER R, EUGSTER M. Environmental impacts of Swiss collection and recovery systems for Waste Electrical and Electronic Equipment (WEEE): A follow-up [J]. Science of the Total Environment, 2011, 409: 1746-1756.
[5] Zied D, Bouda M, Ridha B C, Guy M, Rajeshwa D T,  is B. Development of a new chemical equilibrium and techno-economic model for the treatment of metal-polluted effluents by precipitation techniques [J]. Hydrometallurgy, 2009, 98: 247-256.
is B. Development of a new chemical equilibrium and techno-economic model for the treatment of metal-polluted effluents by precipitation techniques [J]. Hydrometallurgy, 2009, 98: 247-256.
[6] Mellah A, Benachour D. The solvent extraction of zinc and cadmium from phosphoric acid solution by di-2-ethyl hexyl phosphoric acid in kerosene diluent [J]. Hydrometallurgy, 2006, 81: 100-103.
[7] Vijayalakshmi A, Lawrence A D, Nagendran A, Mohan D. Separation of proteins and toxic heavy metal ions from aqueous solution by CA/PA blend ultrafiltration membranes [J]. Separation and Purification Technology, 2008, 62: 32-38.
[8] Ujang Z, Anderson G K. Performance of low pressure reverse osmosis membrane (LPROM) for seperating mono- and divalent ions [J]. Water Science and Technology, 1998, 38: 521-528.
[9] Toraj M, Ahmad M, Mohdata S, Amir R. Modeling of metal ion removal from wastewater by electrodialysis [J]. Separation and PurificationTechnology, 2005, 41: 73-82.
[10] Tuzen M, Saygi K O, Soylak M. Pseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ions [J]. Journal of Hazardous Materials, 2008, 152: 632-639.
[11] ZHANG Z P, WANG Z P, WU Q, ZHANG B G, ZHANG Y N. The application of polytetrafluoroethylene fiber grafted by acrylic acid as a cation exchanger for removing Cu2+ [J]. Acta Polymerica Sinica, 2004, 1: 84-87.
[12]  Recovery of copper by LIX 984NC from electroplating rinse bath solution [J]. Hydrometallurgy, 2009, 98: 86-91.
Recovery of copper by LIX 984NC from electroplating rinse bath solution [J]. Hydrometallurgy, 2009, 98: 86-91.
[13] Tuzen M, Soylak M. Column solid-phase extraction of nickel and silver in environmental samples prior to their flame atomic absorption spectrometric determinations [J]. Journal of Hazardous Materials, 2009, 164: 1428-1432.
[14] Kumar R J F, Radhakrishnan V, HARIDOSS P. Enhanced mechanical and electrochemical durability of multistage PTFE treated gas diffusion layers for proton exchange membrane fuel cells [J]. International Journal Hydrogen Energy, 2012, 37(14): 10830-10835.
[15] Sun H, Sun Z, Wu Y. Proton transfer mechanism in perfluorinated sulfonic acid polytetrafluoroethylene [J]. International Journal Hydrogen Energy, 2012, 37(17): 12821-12826.
[16] XIONG C H, JIA Q, CHEN X Y, WANG G T, YAO C P. Optimization of polyacrylonitrile-2-aminothiazole resin synthesis, characterization, and its adsoption performance and mechanism for removal of Hg(II) from aqueous solution [J]. Industrial & Engineering Chemistry Research, 2013, 52: 4978-4986.
[17] LI S, FENG Y P, FANG L, ZHENG X M, CHEN D C, YAO L Y, XIONG C H. Synthesis and characterization of a novel chloromethylated polystyrene-g-2-adenine chelating resin and its application to preconcentrate and detect the concentration of mercury ions in edible mushroom samples [J]. Candian Journal of Chemistry, 2016, 94: 751-758.
[18] WEI J F, HU R X, ZHANG Z P. Polytetrafluoroethylene ion exchange fibers and its absorption properties of Pb2+ and Cu2+ [J]. Journal of Tianjin Polytechnic University, 2005, 24(1): 4-8. (in Chinese)
[19] Xiong C H, Yao C P. Preparation and application of acrylic acid grafted polytetrafluoroethylene fiber as a weak acid cation exchanger for adsorption of Er(III) [J]. Journal of Hazardous Materials, 2009, 170: 1125-1132.
[20] Langmuir I. The constitution and fundamental properties of solids and liquids [J]. Journal of the Franklin Institute, 1917, 183: 102-105.
[21] Freundlich H M F. Uber die adsorption in losungen [J]. Zeitschrift Fur Physikalische Chemie-International. Journal of Research in Physical Chemistry and Chemical Physics, 1906, 57: 385-470. (in German)
[22]  N, Kavak D. Adsorption of boron from aqueous solutions using fly ash: Batch and column studies [J]. Journal of Hazardous Materials, 2005, 127(B): 81-88.
N, Kavak D. Adsorption of boron from aqueous solutions using fly ash: Batch and column studies [J]. Journal of Hazardous Materials, 2005, 127(B): 81-88.
[23] Xiong C H, Yao C P. Study on the adsorptionofcadmium(II) from aqueous solution by D152 resin [J]. Journal of Hazardous Materials, 2009, 166(2/3): 815-820.
[24] Boyd G E, Adamson A W. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics [J]. Journal of the Chemical Society, 1947, 69: 2836-2848.
[25] Chen Y Y, Zhao Y. Synthesis and characterization of polyacrylonitrile-2-amino-2-thiazoline resin and its sorption behaviors for noble metal ions [J]. Reactive and Functional Polymers, 2003, 55: 89-98.
[26] Aksu Z, Gonen F. Biosorption of phenol onto Posidonia oceanica (L.) seagrass in batch system: Equilibrium and kinetic modeling [J]. Process Biochemistry, 2004, 39(5): 599-613.
[27] Kuang Y, Du J H, Zhou R B. Chen Z L,Meghara j M, Naidu R. Calcium alginate encapsulated Ni/Fe nanoparticles beads for simultaneous removalofCu(II) and monochlorobenzene [J]. Journal of Colloid and Interface Science, 2015, 447: 85-91.
[28] Keller C, Rizwan M, Davidian J C, Pokrovsky O S, Bovet N, Chaurand P, Meunier J D. Effectofsilicon on wheat seedlings (Triticum turgidum L.) growninhydroponics and exposed to 0 to 30 A mu MCu [J]. Planta, 2015, 241(4): 847-860.
(Edited by YANG Bing)
Foundation item: Project(20133326110006) supported by PhD Programs Foundation of Ministry of Education of China; Project(2005002) supported by Zhejiang Provincial Key Laboratory of Fiber Materials and Manufacturing Technology, China; Project(YR2015002) supported by Zhejiang Provincial Top Key Academic Discipline of Chemical Engineering and Technology of Zhejiang Sci-Tech University, China
Received date: 2015-10-04; Accepted date: 2016-01-13
Corresponding author: XIONG Chun-hua, Professor; Tel: +86-571-88932083; Fax: +86-571-88835193; E-mail: xiongch@163.com
Abstract: An enhanced adsorption and desorption procedure of Cu(II) onto green synthesized acrylic acid grafted polytetrafluoroethylene fiber (i.e. AA-PTFE) was conducted with various chemical methods. The results show that the optimal adsorption condition is in acetic acid, sodium acetate (HAc-NaAc) buffer solution (pH=6.80) with the initial concentration of 0.2 mg/mL. The process is very fast initially and equilibrium time is 12 h with a high Cu(II) uptake of 112.26 mg/g at 298 K. Various thermodynamic parameters indicate that the adsorption process is spontaneous and endothermic in nature. In the elution test, 2 mol/L HCl solution achieves satisfactory elution rate and shows no significant decrease after 5 adsorption-desorption cycle, which indicates that AA-PTFE can be regenerated and reused, and due to which a reasonable amount of nondegradable polymer material is avoided in industrial use. Finally, PTFE, AA-PTFE fiber, and Cu(II) loaded AA-PTFE fiber were characterized with various techniques, including IR spectroscopic technique, SEM and EDS.


