
Structure and properties of ceramic coatings formed on
aluminum alloys by microarc oxidation
LIU Wan-hui(刘万辉), BAO Ai-lian(鲍爱莲), LIU Rong-xiang(刘荣祥), WU Wan-liang(武万良)
College of Materials Science and Engineering, Heilongjiang Institute of Science and Technology,
Harbin 150027, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
The thick and hard ceramic coatings were deposited on 2024 Al alloy by microarc oxidation in the electrolytic solution. Microstructure, phase composition and wear resistance of the oxide coatings were investigated by SEM, XRD and friction and wear tester. The microhardness and thickness of the oxide coatings were measured. The results show that the ceramic coating is mainly composed of α-Al2O3 and γ-Al2O3. During oxidation, the temperature in the microarc discharge channel is very high to make the local coating molten. From the surface to interior of the coating, microhardness increases gradually. The microhardness of the ceramic coating is HV1 800, and the microarc oxidation coatings greatly improve the antiwear properties of aluminum alloys.
Key words:
2024 alloy; ceramic coatings; aluminum alloys; microarc oxidation; wear resistance; mechanical properties;
1 Introduction
Aluminum alloys are characterized by low density and high ratio of strength to mass, therefore, they are used in variety of technical applications such as aerospace, power engineering, vehicle and civil architecture and structural fields. However, the application of aluminum alloys is limited because of their extreme poor surface performance, although these materials are of increasing interest in aerospace, automotive and textile engineering due to their lightness. The protection of aluminum alloys from wear by applying coatings of ceramic materials is currently of great interest. A number of techniques have been applied to produce thick ceramic coating on aluminum alloy substrate[1-4]. Microarc oxidation (MAO), also called plasma electrolytic oxidation, has attracted considerable attention in recent years as an environmentally friendly alternative to produce coatings with superior resistance to wear and corrosion. The microarc oxidation process has been developed to provide modified surfaces with improved load support on Al, Mg, Ti and their alloys, and protect them from severe wear and corrosion. It has been known that the sparking reaction due to dielectric breakdown leads to the oxide ceramic deposition of material from an aqueous electrolyte[3-9]. The oxidized coatings have good wear and corrosion resistance, high microhardness and good adhesion with substrate. In this paper, a ceramic coating with a thickness of about 50 μm was directly formed on the surface of 2024 aluminum alloy through microarc oxidation in an electrolytic solution in order to improve its surface property greatly. The microstructure, phase composition, and mechanical properties of oxide coatings were analyzed.
2 Experimental
Ceramic coatings were prepared on 2024 aluminum alloy (Si 0.5%, Fe 0.5%, Cu 3.8%-4.4%, Mn 0.3%-0.9%, Mg 1.2%-1.8%, Zn 0.3%, balance Al; roughness Ra=(1.5±0.3)μm) samples with a 30 kW microarc oxidation equipment consisting of a potential adjustable AC power supply. The electrolytic solution was composed of KOH and Na2SiO3 in distilled water. The solution temperature was kept below 40 ℃ during oxidation and the current density on the sample surface was controlled at 10 A/dm2. The deposition process was stopped when the coating thickness comes to an appropriate value. The oxidation process is shown in Fig.1. The thickness of the microarc oxidation coatings was measured with a MINITEST 1100 microprocessor coating thickness gauge based on the eddy current technique.

Fig.1 Schematic diagram of experimental arrangement of microarc oxidation: 1 Power supply; 2 Electrolyzer; 3 Thermo- meter; 4 Sample; 5 Stirrer; 6 Cooling system
The morphology of the oxide coating was observed with a MS-2600 scanning electron microscope(SEM). The elemental composition of the oxide coating was determined using X-ray energy dispersive spectrometer (EDS) attached to the scanning electron microscope. The crystallographic characteristics of the coatings were investigated on an XD-2500 X-ray diffractometer (Cu Kα radiation). The microhardness of the oxide layers was measured with an MXT-1 electronic microhardness tester. The wear resistance of the ceramic layers was evaluated on an MMS-2B ball-on-disk friction and wear tester, using d 4 mm GCr15 steel with the hardness of HRC62 as the counterpart. The load and sliding speed was 10 N and 0.2 m/s, respectively. Diameter of wear track on disk was 15 mm.
3 Results and discussion
3.1 Surface morphology
Fig.2 reveals the surface morphology of microarc oxidation coatings on 2024 aluminum alloy. After oxidation treatment, the samples’ surfaces become rough. The surface of microarc oxidation coatings contains many grains with various sizes, and the grains contact with each other, as shown in Fig.2(a). During microarc discharge reaction, many visible spark or microarc spots move quickly on the surface of aluminum alloy. The microarc discharge over several times at the same position of sample would result in one large grain due to the deposition of eruptive melt from the discharge channel[10-11].
Fig.2(b) shows one crater-like hole of several micrometers remains at the center of grain. The trace of rapid solidification of oxide melt can be clearly observed on this large grain surface around the center of the discharge hole. It can be known that the instantaneous temperature in the microarc discharge zone reaches several thousand degrees centigrade and the coatings are locally molten[9-12]. In addition, there are also many small holes of less than 1 μm in diameter on the surface, which correspond to the fine sparks of specimen surface during oxidation, but these holes do not penetrate the whole coating.
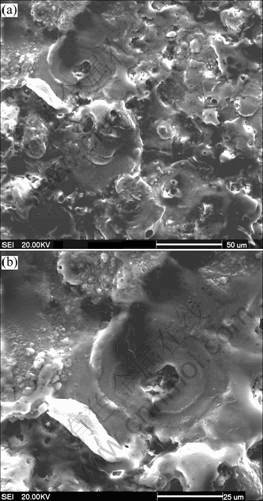
Fig.2 Surface morphologies of micro-arc oxidized ceramic coating: (a) Low magnification; (b) High magnification
3.2 Cross-sectional morphology
Fig.3 shows the cross-sectional micrographs of the ceramic coating formed on the surface of 2024 aluminum alloys. Microarc oxidation coatings grow into the metal substrate to form the ceramic layer with better properties. It indicates that the oxide coating actually contains two layer including dense layer and loose layer. The average thickness of the microarc oxidation ceramic coating is measured by an eddy current technique. The whole thickness is about 55 μm. The MAO coating obtained at a typical current density was polished to remove the loose layer, and then the hardness of the dense was measured. The surface hardness of the dense layers was measured with an MXT-1 electronic micro-hardness tester at a load of 0.98 N. The cross-section hardness of the coatings was also measured in the same apparatus. The dense coatings hardness increases gradually with increasing coating thickness, even above HV0.11 800. This might be attributed to the changes in the phase composition and porosity of the coatings along the depth. When the oxide coating increases, the phase change in dense layer has reinforced and the content of phase increases, so the hardness of dense layer surface becomes larger with increasing thickness. It is known that the hardness and α-Al2O3 contents of the coatings show content corresponding to the maximal hardness. In other words, an increase in the contents of γ-Al2O3 leads to a decrease in hardness.

Fig.3 Cross-sectional morphology of MAO ceramic coatings: Ⅰ Substrate; Ⅱ Dense layer; Ⅲ Loose layer
3.3 XRD analysis
Fig.4 shows the X-ray diffraction pattern of the ceramic coating. It indicates that the ceramic coatings contain mainly α-Al2O3, γ-Al2O3 phases and substrate aluminum. The α-Al2O3 content in the dense layer is higher than that in the loose layer. This is mainly caused by variation in cooling rate of the molten alumina in the microarc zone. The growth of oxide coating mainly depends on the combination between Al and O in microarc oxidation process. The inner layer retains higher temperature which is high enough to transform γ-Al2O3 to α-Al2O3, owing to low thermal conductivity of the alumina oxide. Thus, a considerably increase of wear resistance for aluminum alloys after microarc oxidation treatment could be attributed to the formation of hard ceramic coatings.
3.4 Friction and wear behaviors
After microarc oxidation processing, wear resistance of the specimen is far better than the untreated specimen. Figs.5(a) and (b) show that the microarc oxidation samples have higher wear resistance than the untreated aluminum alloy samples, which indicates that the wear resistance of Al alloy could be improved obviously by microarc oxidation. The wear resistance of the ceramic layer is relevant to the surface quality, phase composition and microstructure. Under the wear and friction condition, α-Al2O3 bears most of the load because of its relatively high hardness. The friction coefficient changing with the increase of the sliding distance is of regularity for microarc oxidation coating under dry friction condition against GCr15 ball with hardness of HRC62. The primary wear mechanism of oxidation coatings against GCr15 ball is also surface fracture. The fine surface and suitable microstructure of the microarc oxidation ceramic layer obtained on 2024 aluminum alloys result in excellent wear resistance.
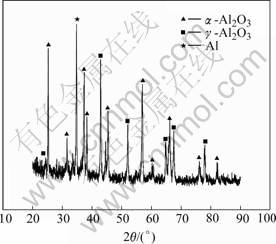
Fig.4 XRD pattern of ceramic coatings formed on 2024 Al by microarc oxidation
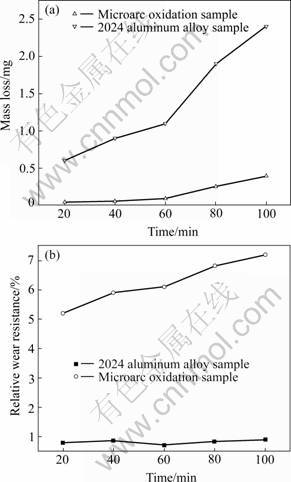
Fig.5 Mass loss under wearing (a) and relative wear resistance (b) for Al substrate and microarc oxidation coating thereon
4 Conclusions
1) The micro-arc oxidation coatings are divided into the loose superficial layer and the inner dense layer and they are mainly composed of α-Al2O3 and γ-Al2O3.
2) The micro-hardness of dense coatings increases gradually with increasing coating thickness, even above HV0.11 800.
3) The oxidation ceramic coatings have greatly improved the antiwear properties of aluminum alloys with microarc oxidation techniques.
References
[1] WEI Tong-bo, YAN Feng-yuan, LIU Wei-min, TIAN Jun. Structure and tensile/wear properties of microarc oxidation ceramic coatings on aluminum alloy [J]. Trans Nonferrous Met Soc China, 2004, 14(6): 1162-1169.
[2] WANG Li-shi, CAI Qi-zhou, WEI Bo-kang, LIU Quan-xin. Electrochemical performance of microarc oxidation films formed on AZ91D alloy in two group electrolytes [J]. Trans Nonferrous Met Soc China, 2005, 15 (4): 783-777.
[3] LUO Zhuang-zi, ZHANG Zhao-zhu, LIU Wei-min, TIAN Jun. Tribological properties of solid lubricating film/microarc oxidation coating on Al alloys [J]. Trans Nonferrous Met Soc China, 2005, 15(6): 1231-1236.
[4] XUE Wen-Ben, WANG Chao, CHEN Ru-yi, DENG Zhi-wei. Structure and properties characterization of ceramic coatings produced on Ti-6Al-4V alloy by microarc oxidation in aluminate solution [J]. Material Letter, 2002, 52: 435-441.
[5] XUE Wen-bin, WANG Chao, LI Yong-liang, CHEN Ru-yi. Charaterization of microarc oxidation coatings on pure titanium [J]. Rare Metals, 2003, 22(1): 42-47.
[6] NIE X, LEYLAND A, MATTHEWS A. Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of microarc [J]. Surface and Coatings Technology, 2000, 125: 407-412.
[7] XUE W B, DENG Z W, CHEN R Y. Microstructure and properties of ceramic coatings produced on 2024 aluminium alloy by microarc oxidation [J]. Journal of Materials Science, 2001, 36(3): 2615-2621.
[8] YAO Zhong-ping, JIANG Zhao-hua, SUN Xue-tong, et al. Composition and structure of coatings on Ti-6Al-4V alloy by micro-plasma oxidation in NaAlO2 solution [J]. Trans Nonferrous Met Soc China, 2004, 14(1): 162-165.
[9] YUAN Yang, LUO Zhuang-zi, TIAN Jun. The antiwear and lubrication coating produced by microarc oxidation on Al alloy [J]. Materials Protection, 2002, 35(5): 4-6. (in Chinese)
[10] WANG Hui, WANG Hao-wei. Thick and hard anodized aluminum film with large pores for surface composites [J]. Trans Nonferrous Met Soc China, 2004, 14(1): 135-142.
[11] LUO Zhuang-zi, ZHANG Zhao-zhu, LIU Wei-min, et al. The overcoat oil lubrication of microarc oxidation coating on Al alloy by liquid plasma technique [J]. Materials Protection, 2004, 37: 33-36.
[12] TIAN Jun, LUO Zhuang-zi, QI Shang-kui, et al. Structure and antiwear behavior of microarc oxidized coatings on aluminum alloy [J]. Surface and Coatings Technology, 2002, 3: 1-7.
(Edited by HE Xue-feng)
Foundation item: Project(04-71) supported by the Scientific Research Startup Foundation of Heilongjiang Institute of Science and Technology, China
Corresponding author: LIU Wan-hui; Tel: +86-451-88616478; E-mail: wanhui7468@126.com


