Trans. Nonferrous Met. Soc. China 30(2020) 1697-1706
Synthesis of high-purity ultrafine tungsten and tungsten carbide powders
Kai-fei WANG, Guo-hua ZHANG
State Key Laboratory of Advanced Metallurgy, University of Science and Technology Beijing, Beijing 100083, China
Received 20 September 2019; accepted 13 April 2020
Abstract:
High-purity ultrafine W or WC powder was prepared via a two-step process composed of the carbothermic pre-reduction of WO2.9 and the following deep reduction with H2 or carbonization with CH4+H2 mixed gases. The effects of C/WO2.9 molar ratio and temperature on phase composition, morphology, particle size, and impurity content of products were investigated. The results revealed that when the C/WO2.9 ratio was in the range from 2.1:1 to 2.5:1, the carbothermic pre-reduction products consisted of W and a small amount of WO2. With changing C/WO2.9 ratio from 2.1:1 to 2.5:1, the particle sizes were gradually decreased. In order to prepare ultrafine W or WC powder, a relatively high C/WO2.9 ratio and a lower reaction temperature at this stage were preferred. After the second reaction, the final products of ultrafine W and WC powders with a high purity could be obtained, respectively.
Key words:
carbothermic pre-reduction; carbonization; tungsten; tungsten carbide;
1 Introduction
Tungsten and tungsten carbide have many unique properties, such as high melting point, high thermal stability, superior hardness, high oxidation resistance and corrosion resistance, and good electrical conductivity [1-6]. Due to these excellent properties, W and WC have been widely used in the fields of aerospace, military, metallurgy, cutting, mining tools and drilling tools etc [7-14].
Ultrafine tungsten and tungsten carbide powders have many potential applications compared to traditional tungsten and tungsten carbide, which could greatly improve the physical, chemical and mechanical properties of W alloys and WC-Co hard metals [12,13]. Therefore, the preparation of them becomes the current research focus. Table 1 gives the methods for preparation of W and WC in the last twenty years [8-17]. Among them, the widely used methods for industrial preparations of W and WC are hydrogen reduction of tungsten oxide (WO3, WO2.9) to prepare W powder, and further carbonization of W to produce WC [16,18]. It has been reported that owing to the generation of gaseous hydroxides (WO2(OH)2) during the hydrogen reduction process of tungsten oxide, the transport of W is enhanced by chemical vapor transport (CVT) mechanism [12-14,16,18], which could lead to the formation of W particle with a large size. Meanwhile, long reaction time and high temperature during the carburization stage were also needed due to slow kinetics of solid-solid reaction between W and C to generate WC. Therefore, it was very difficult to produce the ultrafine W and WC powders.
In order to weaken the influence of CVT mechanism and high temperature on the particle size of products, we have reported a new carbothermic reduction method to remove the oxygen from metal oxides (WO3, MoO3) by reacting with nano carbon black in order to prepare ultrafine Mo, W and WC [12,16,18]. Compared with the hydrogen reduction, the carbothermic reduction has many advantages, such as low requirement for the equipment, simple operation, and low price of reductant. However, it is widely recognized that carbothermic reduction always leads to a high content of residual free carbon in the product [18,19]. In this study, the high-purity ultrafine W and WC powders are prepared based on the carbothermic reduction process, as approximately described in Fig. 1. First, the commercial blue tungsten oxide (WO2.9), as the tungsten source, reacted with insufficient carbon black to remove most of oxygen from WO2.9. After that, the deep reduction or carburization process was conducted to prepare tungsten and tungsten carbide at a low temperature. The phase transition, morphology evolution, particle size, carbon and oxygen contents of product at different stages are investigated in details.
Table 1 Methods for preparations of W and WC
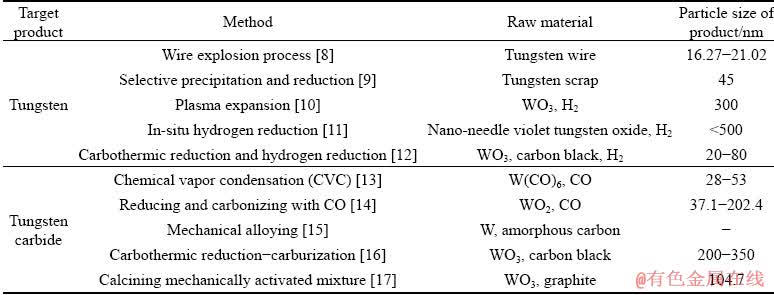

Fig. 1 Process flow chart of new carbothermic reduction method
2 Experimental
Commercial blue tungsten oxide (WO2.9) powder from Ganzhou Huaxing Tungsten Products Co., Ltd. (China) was used as the tungsten source. Carbon black purchased from Mitsubishi Chemical Corporation was used as the main reduction agent. The characterization of carbon black, schematic diagram of experimental apparatus and the experimental procedures were shown in our previous researches [16,18]. In the carbothermic pre-reduction stage, nitrogen (N2, 99.999%) was used as protection gas under a constant gas flow rate of 300 mL/min. Hydrogen (H2, 99.999%) and methane (CH4, 99.999%) were used as the gaseous reducing and carburizing agent for preparing W and WC at the second stage, respectively. In this study, four groups of experiments with different C/WO2.9 molar ratios (2.1:1, 2.3:1, 2.5:1 and 2.7:1) were investigated in order to study the effect of C/WO2.9 ratio on the phase transition and morphology evolution of the products. The WO2.9 and carbon black were carefully weighed based on the specified C/WO2.9 ratio and uniformly mixed by agate mortar.
The phase compositions and morphology of the samples were characterized by X-ray diffraction (XRD; TTR III, Rigaku Corporation, Japan), field-emission scanning electron microscopy (FE-SEM; ZEISS SUPRA 55, Oberkochen, Germany), respectively. The infrared oxygen- nitrogen-hydrogen analyzer (EMIA-920V2, HORIBA, Japan) and infrared carbon-sulfur analyzer (EMIA-920V2, HORIBA, Japan) were used to measure the oxygen and carbon contents of final products, respectively.
3 Results and discussion
3.1 Carbothermic pre-reduction process
3.1.1 Non-isothermal experiments
To obtain the suitable reaction temperature of first carbothermic pre-reduction stage, a non-isothermal experiment with a ramping rate of 5 °C/min was performed in a thermal analysis system (HTC-2, Beijng Hengjiu Instrument Ltd. China), and the thermo-gravimetric differential thermal analysis (TG-DTA) curves of 90 mg as-milled powder (with the composition of WO2.9 + 2.5C) are shown in Fig. 2. According to the TG curve, two turning points appeared at about 925 and 1081 °C, respectively, which indicated that the temperature required for carbothermic reduction of blue tungsten oxide began at above 925 °C, and finished at 1081 °C. From the DTA curve, three endothermic peaks were detected in the whole carbothermic pre-reduction process, indicating that three main reactions occur during this process. Therefore, to ensure a large reaction rate, the temperatures for the first stage were determined to be 1050 and 1150 °C.
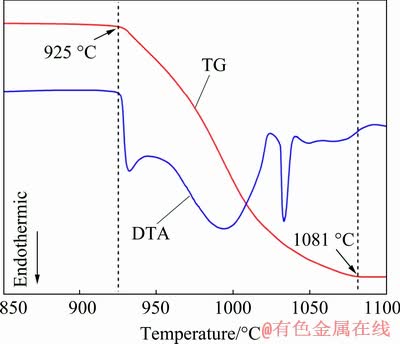
Fig. 2 TG-DTA curves of as-milled powder of WO2.9 + 2.5C at heating rate of 10 °C/min
3.1.2 Phase transition and microstructure evolution
In order to investigate the phase transition and microstructure evolution during the carbothermic pre-reduction process, the sample with C/WO2.9 molar ratio of 2.5:1 was reacted at 1050 °C for different time under nitrogen atmosphere. After that, the sample was cooled down to room temperature before being taken out from the quartz tube. Figure 3 presents XRD patterns of the carbothermic pre-reduction products prepared after different reaction time. When the reaction time was 10 min, it could be seen that all the diffraction peaks were assigned to WO2.72, WO2 and W, but no peak belonging to WO2.9 was detected. Therefore, it could be concluded that WO2.9 was easily reduced to WO2.72. When the reaction time was 20 min, the relative diffraction intensity of WO2.72 gradually decreased, but the intensity of W and WO2 increased. As the time was prolonged to 30 min, 1 h and 2 h, the diffraction peaks attributable to WO2.72 disappeared, and all the peaks were identified to WO2 and W. Furthermore, the peak intensity of W and WO2 gradually increased and decreased, respectively. However, there was still a small amount unreacted WO2 even if the reaction time was 2 h, since the content of carbon black was not sufficient to reduce all the tungsten oxide at low C/WO2.9 molar ratio of 2.5:1. Therefore, the total reactions can be described as follows: WO2.9→ WO2.72→WO2→W.
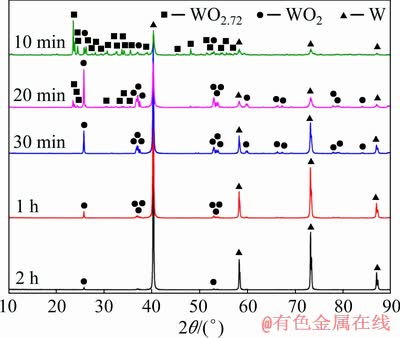
Fig. 3 XRD patterns of carbothermic pre-reduction products obtained after reacting at 1050 °C for different time with C/WO2.9 molar ratio of 2.5:1
The morphologies of raw material WO2.9 and carbothermic pre-reduction products obtained at 1050 °C after reacting for different time for sample with C/WO2.9 ratio of 2.5:1 were also detected by field-emission scanning electron microscopy (FE-SEM), and the images are shown in Fig. 4. From Figs. 4(a1, a2), it could be known that the large WO2.9 particles were irregular blocky-shaped with the average size of about 55 μm, which were composed of many platelet-shaped grains with the size of about 70 nm. After reacting for 10 min, from Figs. 4(b1, b2), it could be seen that lots of grains were needle-shaped with the average size of above 1 μm. According to the literatures [11,20,21], the needle-shaped product was WO2.72, which coincided with the XRD results shown in Fig. 3. When the reaction time was increased to 20 and 30 min, as shown in Figs. 4(c, d), the large particle with needle-like shape disappeared, and all particle size became smaller than 100 nm. As further prolonging the reaction time to 1 h, it could be seen that the products were composed of a large number of smaller particles with irregular shape and few larger particles with smooth surface, which had the particle size of ~70 and 500 nm, respectively. Combined with the XRD results shown in Fig. 3, it could be concluded that the two kinds of particles were WO2 and W, respectively. Finally, the uniform and highly-dispersed powder was obtained when the reaction time was 2 h.
Based on the above experimental results, a holding time of 2 h was used for the carbothermic pre-reduction stage. 10 g mixed powders with different C/WO2.9 molar ratios were roasted at the target temperature in flowing N2 gas atmosphere, and the phase compositions are shown in Fig. 5. It could be seen that regardless of the experimental conditions, the main phases of products were W and a small amount of WO2 expect the case of C/WO2.9 ratio of 2.7:1. With the increase of the C/WO2.9 molar ratio from 2.1:1 to 2.5:1, the peak intensity of WO2 was gradually decreased, but that of the W was increased. In addition, when the C/WO2.9 molar ratio was a constant, the peak intensity of WO2 was decreased with the increase in temperature. It could be concluded that a higher C/WO2.9 molar ratio and a higher temperature are beneficial to the reduction of tungsten oxide to tungsten. When the C/WO2.9 ratio was increased to 2.7:1, it was noted that no diffraction peak of tungsten oxide was detected. Besides the main phase W, a new phase W2C appeared, which indicated that a C/WO2.9 ratio of 2.7:1 was enough to remove all the oxygen from the raw material WO2.9.
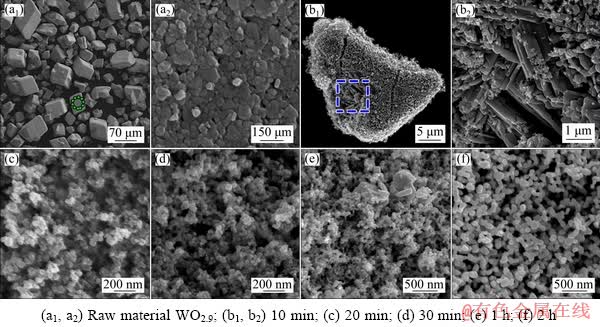
Fig. 4 FE-SEM images of raw materials of WO2.9 and carbothermic pre-reduction products obtained after reacting at 1050 °C for different time with C/WO2.9 molar ratio of 2.5:1
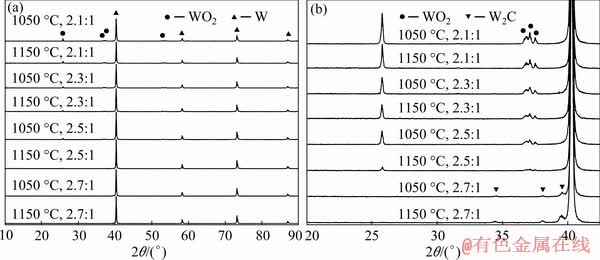
Fig. 5 XRD patterns of carbothermic reduction product prepared at 1050 and 1150 °C for 2 h with different C/WO2.9 ratios
Figures 6(a-h) show the FE-SEM images of the carbothermic pre-reduction products obtained after reacting for 2 h. The particle sizes were estimated from three FE-SEM images and the average particle sizes for sample reacting under different conditions are shown in Fig. 6(i). From Figs. 6(a-h), it could be seen that most of the particles had smooth and irregular shape in all the cases. In addition, as the C/WO2.9 ratio was increased from 2.1:1 to 2.5:1, the average particle size was decreased from 112 to 58 nm at 1050 °C and from 163 to 76 nm at 1150 °C, respectively. Furthermore, it could be also noted that with the increase of carbon content, the carbothermic pre-reduction products had higher dispersion and looser structure. In the previous study to prepare Mo, W and WC powders [12,16,18, 22,23], it was found that relatively high carbon content was beneficial to decreasing the particle size of product. The same conclusion was also obtained in the current study. However, when the ratio of C/WO2.9 was further increased to 2.7:1, the particles had obviously grown up by neck-sintering and the mean particle sizes were increased to 141 nm at 1050 °C and 224 nm at 1150 °C. Combined with the phase compositions of products at C/WO2.9 ratio of 2.7:1, the reason for this phenomenon may be that W2C which has a lower melting point relative to W was generated at a C/WO2.9 ratio of 2.7:1, which leads to a higher sintering activity of products to promote the particle growth. Moreover, when the reaction temperature was increased from 1050 to 1150 °C at a constant ratio of C/WO2.9, the particle size was increased in all the cases, since a high temperature was beneficial to the sintering process. This further confirmed that the C/WO2.9 ratio and the reaction temperature had significant effects on the particle size of carbothermic pre-reduction product.

Fig. 6 FE-SEM images (a-h), average particle size (i), and carbon and oxygen contents (j) of carbothermic pre-reduction products obtained at different temperatures and C/WO2.9 ratios
The oxygen and carbon contents of the carbothermic pre-reduction products obtained at 1050 °C were measured, and the results are shown in Fig. 6(j). It could be seen that with the increase of C/WO2.9 molar ratio, the carbon content gradually increased from 0.019% to 0.089%, but the oxygen content decreased from 4.84% to 0.098%. Combined with the XRD results (Fig. 5), owing to the presence of tungsten oxide (WO2), the product with C/WO2.9 ratio of 2.1:1 had a relatively high oxygen content and low carbon content. However, for the C/WO2.9 ratio of 2.5:1, the values of oxygen content and carbon content were 0.735% and 0.031%, respectively, which indicated that a molar ratio of 2.5:1 was very close to the theoretical stoichiometric ratio of preparing W. As further increasing C/WO2.9 ratio to 2.7:1, the carbon content in the product increased to 0.089%, while the oxygen content decreased to 0.098%. The reason for this was that the sufficient carbon could remove all the oxygen from tungsten oxide, but the excessive carbon could react with W to generate W2C. It was pointed out that the tungsten oxide has great potential for removing W2C and free carbon [12]. Therefore, in order to prepare high- purity ultrafine W and WC powders, a relatively low C/WO2.9 ratio of 2.1:1 or 2.5:1 should be adopted in the first stage.
3.2 Preparation of W powder by deep reduction with H2
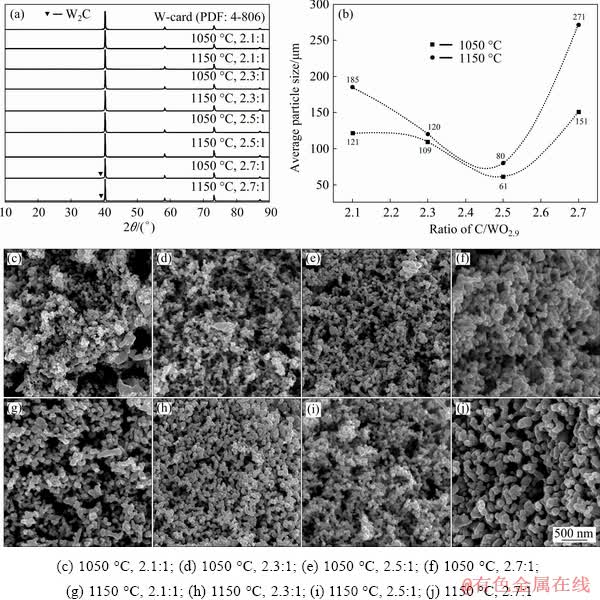
Fig. 7 XRD patterns (a), average particle size (b) and FE-SEM images (c-j) of products after deep reduction at 750 °C for 2 h
To remove the residual oxygen and prepare high purity tungsten powder, the carbothermic pre-reduction products were deeply reduced by hydrogen at a low temperature of 750 °C for 2 h. The XRD patterns of secondary reduction product are shown in Fig. 7(a), from which it could be noted that all the diffraction peaks were identified to W (PDF Number: 4-806) except the case with a C/WO2.9 ratio of 2.7:1. The oxygen content of the final product was <0.2%, which indicated that the residual tungsten oxides in the carbothermic pre-reduction products could be effectively removed. Furthermore, the carbon contents of final products were also measured by the infrared carbon-sulfur analyzer, which were almost the same with the values in the carbothermic pre-reduction products after the first stage. It could be concluded that the H2 gas could not react with W2C to remove the carbon when the C/WO2.9 ratio was 2.7:1. Therefore, in order to prepare the high-purity W powder with a low carbon content, the carbothermic pre-reduction products with a low residual carbon content (C/WO2.9 ratio <2.7:1) should be adopted. The average particle size and morphologies of W particle after the deep reduction at 750 °C are shown in Figs. 7(b-j). It could be seen that most of the particle had a round shape and smooth surface. With the increase of C/WO2.9 molar ratio from 2.1:1 to 2.5:1, the average particle size decreased from 121 to 61 nm at 1050 °C and from 185 to 80 nm at 1150 °C. However, as the ratio of C/WO2.9 was further increased to 2.7:1, the average particle size of final products was increased to 151 nm at 1050 °C and 271 nm at 1150 °C. Compared with the particle sizes of carbothermic pre-reduction product (Fig. 6(i)), it could be found that the variation trend of the particle size of prepared W was similar to that of carbothermic pre- reduced products after the first stage. In addition, with the increase of C/WO2.9 ratio, the W particle became more loose and uniform. When the C/WO3 molar ratio was 2.5:1, the particle size of the final products (after carbothermic pre-reduction at 1050 or 1150 °C for 2 h, and then hydrogen treatment at 750 °C for 2 h) could reach nanometer scale.
3.3 Preparation of WC by further carburization with CH4-H2 mixed gases
According to Ref. [23], micron-sized tungsten carbide powder could be prepared by reducing ammonium paratungstate and tungsten oxide with mixed gases of H2-CH4. In the current study, in order to convert the residual tungsten oxide, tungsten or carbon-deficient phase (W2C) in the carbothermic pre-reduction products (obtained at C/WO2.9 molar ratio range of 2.1:1-2.7:1) to WC, the products were further carburized by mixed gases of 10%CH4+90%H2. Based on the results of carbothermic pre-reduction stage, it could be concluded that the grain growth by CVT mechanism in the carburization process would be weakened owing to the low content of residual oxygen in carbonthermic pre-reduction product. Figure 8(a) presents the XRD patterns of products after being carburized by mixed gases of 10%CH4+ 90%H2 at 900 °C. It could be clearly seen that no diffraction peaks of tungsten oxide or carbon- deficient phase were detected, and all the peaks were identified to be WC (PDF: 51-939). In addition, all the main peaks of WC were very strong and broad, which suggested that the WC powder obtained had high crystallinity and small particle size.
The average particle size and morphologies of WC obtained after reacting at 900 °C for 1 h are shown in Figs. 8(b-j). It could be seen that there were no obvious changes of morphology for most of the particles relative to carbothermic pre- reduction products. With the C/WO2.9 ratio increasing from 2.1:1 to 2.5:1, the average particle size decreased from 172 to 75 nm at 1050 °C and from 213 to 96 nm at 1150 °C, respectively. When the ratio of C/WO2.9 was further increased to 2.7:1, the average particle size of final products was also increased to 218 nm at 1050 °C and 283 nm at 1150 °C. Combined with the results of average particle sizes of carbothermic pre-reduction products and deep reduction products (W), it could be found that the variation trends of particle sizes for three different products (carbothermic reduction products, W and WC) were similar. Therefore, it could be concluded that the first stage had a crucial effect on the morphology and particle size of the finally prepared W and WC. Furthermore, from the particle sizes of three different products (Figs. 6(i), 7(b) and 8(b)), under the conditions of a specific C/WO2.9 ratio and a specific reaction temperature at first stage, taking 2.3:1 and 1050 °C for instance, the particle sizes of carbothermic reduction product, and the finally obtained W and WC powders were 107, 109 and 150 nm, respectively. Thus, the particle size growth trend from carbothermic pre-reduction product to WC was obviously greater than that to W. This phenomenon may be caused by different reaction temperature, melting point, molar volume of final products. In the current work, the reaction temperatures of preparing W and WC at the second stage were 750 and 900 °C, respectively. It was known that a high temperature promoted the grain growth, while a low temperature was beneficial to preparing products with a relatively small particle size. Meanwhile, W has a much higher melting point than WC (the melting points of W and WC are 3430 and 2850 °C, respectively), which leads to a higher sintering activity of WC powder. Furthermore, WC has a larger molar volume than W, which are 12.53 and 9.52 cm3/mol, respectively [16,18]. Consequently, the prepared WC has a much larger particle size than W. Finally, the carbon contents of those prepared WC were measured, which were in the range of 6.09%-6.17%, which were very close to the theoretical carbon content of WC (6.13%). Therefore, it could be concluded that the high-purity ultrafine WC powder was successfully prepared by two-step process of carbothermic pre-reduction and carburization by CH4-H2 mixed gas.

Fig. 8 XRD patterns (a), average particle size (b) and FE-SEM images (c-j) of carburized products obtained after reacting at 900 °C for 1 h
3.4 Preparation of W and WC by direct reaction between WO2.9 and H2 or 10%CH4+90%H2 mixed gases

Fig. 9 XRD patterns and FE-SEM images of W (a1, a2, a3) and WC (b1, b2, b3) obtained by direct reduction of WO2.9 by H2 or 10%CH4+90%H2 mixed gases
W and WC could also be prepared by directly reacting WO2.9 with H2 and 10%CH4+90%H2 mixed gases. The XRD patterns and FE-SEM images of products after the hydrogen reduction at 750 °C and reduction-carburization at 900 °C by CH4+H2 are shown in Fig. 9, from which it could be found that the all the diffraction peaks were identified to be W and WC, and the products always had a larger particle size (average particle sizes were about 517 nm and 1.43 μm, respectively), relative to these prepared by the current two-stage route. The season for this was that during the reduction process by H2 and CH4+H2 mixed gases, the gaseous hydroxides (WO2(OH)2) were generated, which could significantly accelerate the growth of W particles by chemical vapor transport (CVT) mechanism [12-14,16,18]. Therefore, it could be concluded that the ultrafine W and WC powders are difficult to be prepared by directly using H2 and CH4+H2 mixed gases as the reducing agent. In the current study, the carbothermic pre-reduction process was used for pre-treatment to remove most of oxygen from WO2.9, and then the products further reacted with H2 and CH4+H2 mixed gases to prepare ultrafine W and WC. According the experimental results, it could be concluded that the ultrafine even nano-scale W and WC powders were successfully prepared by this two-step way.
4 Conclusions
(1) The C/WO2.9 molar ratio and temperature at the carbothermic reduction stage had a great effect on the particle size of the products. With the increase of C/WO2.9 ratio from 2.1:1 to 2.5:1 and the decrease of reaction temperature, the particle size of carbothermic pre-reduction product gradually decreased.
(2) The reaction sequence of blue tungsten oxide and carbon black at 1050 °C was WO2.9→ WO2.72→WO2→W.
(3) The carbothermic pre-reduction step was crucial for the control of particle size and impurity of the final product.
(4) The ultrafine or even nano-sized W and WC powders with low contents of impurities were successfully prepared by the two-step process of carbothermic pre-reduction and carburization by CH4+H2 mixed gas.
References
[1] WANG Hong-tao, FANG Z Z, HWANG K S, ZHANG Hai-bo, SIDDLE D. Sinter-ability of nanocrystalline tungsten powder [J]. International Journal of Refractory Metals and Hard Materials, 2010, 28: 312-316.
[2] LI Ping, Sun Da-zhi, WANG Xue, XUE Ke-min, HUA Rui, WU Yu-cheng. Microstructure and thermal stability of sintered pure tungsten processed by multiple direction compression [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 461-468.
[3] SUN Ya-li, LIU Qing-cai, HUANG Xin, ZHANG Fa-xing, YANG Jian, MEI Hua. Effect of jet milling on micro-strain behavior and rupture behavior of agglomerates of ultrafine WC powders [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(10): 2128-2140.
[4] REN Chai, FANG Z Z, ZHANG Huan, KOOPMAN M. The study on low temperature sintering of nano-tungsten powders [J]. International Journal of Refractory Metals and Hard Materials, 2016, 61: 273-278.
[5] GUO Sheng-da, BAO Rui, YANG Ping, Yi Jian-hong. Morphology and carbon content of WC-6%Co nanosized composite powders prepared using glucose as carbon source [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(4): 722-728.
[6] REN Chai, FANG Z Z, KOOPMAN M, BUTLER B, PARAMORE J, MIDDLEMAS S. Methods for improving ductility of tungsten—A review [J]. International Journal of Refractory Metals and Hard Materials, 2018, 75: 170-183.
[7] LIU Xue-mei, WANG Hai-bin, SONG Xiao-yan, MOSCATELLI R. Elastic modulus of nanocrystalline cemented carbide [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(5): 966-973.
[8] SARATHI R, SINDHU T K, CHAKRAVARTHY S R, SHARMA A, NAGESH K V. Generation and characterization of nano-tungsten particles formed by wire explosion process [J]. Journal of Alloys and Compounds, 2009, 475: 658-663.
[9] KAMAL S S K, SAHOO P K, VIMALA J, SHANKER B, GHOSAL P, DURAI L. Synthesis of high purity tungsten nanoparticles from tungsten heavy alloy scrap by selective precipitation and reduction route [J]. Journal of Alloys and Compounds, 2016, 678: 403-409.
[10] SARMAH T, AOMOA N, BHATTACHARJEE G, SARMA S, BORA B, SRIVASTAVA D N, BHUYAN H, KAKATI M, TEMMERMAN G D. Plasma expansion synthesis of tungsten nanopowder [J]. Journal of Alloys and Compounds, 2017, 725: 606-615.
[11] WU Chong-hu. Preparation of ultrafine tungsten powders by in-situ hydrogen reduction of nano-needle violet tungsten oxide [J]. International Journal of Refractory Metals and Hard Materials, 2011, 29: 686-691.
[12] SUN Guo-dong, WANG Kai-fei, SONG Cheng-min, ZHANG Guo-hua. A low-cost, efficient, and industrially feasible pathway for large scale preparation of tungsten nanopowders [J]. International Journal of Refractory Metals and Hard Materials, 2019, 78: 100-106.
[13] KIM J C, KIM B K. Synthesis of nanosized tungsten carbide powder by the chemical vapor condensation process [J]. Scripta Materialia, 2004, 50: 969-972.
[14] DANG Jie, WU Yi-jie, LV Ze-peng, LV Xue-wei. Preparation of tungsten carbides by reducing and carbonizing WO2 with CO [J]. Journal of Alloys and Compounds, 2018, 745: 421-429.
[15] BOLOKANG S, BANGANAYI C, PHASHA M. Effect of C and milling parameters on the synthesis of WC powders by mechanical alloying [J]. International Journal of Refractory Metals and Hard Materials, 2010, 28: 211-216.
[16] WANG Kai-fei, SUN Guo-dong, WU Yue-dong, ZHANG Guo-hua. Fabrication of ultrafine and high-purity tungsten carbide powders via a carbothermic reduction–carburization process [J]. Journal of Alloys and Compounds, 2019, 784: 362-369.
[17] MA J, ZHU S G. Direct solid-state synthesis of tungsten carbide nanoparticles from mechanically activated tungsten oxide and graphite [J]. International Journal of Refractory Metals and Hard Materials, 2010, 28: 623-627.
[18] WANG Kai-fei, SUN Guo-dong, WU Yue-dong, ZHANG Guo-hua. Size-controlled synthesis of high-purity tungsten carbide powders via a carbothermic reduction-carburization process [J]. International Journal of Refractory Metals and Hard Materials, 2019, 84: 104975.
[19] WANG Da-hang, ZHANG Guo-hua, CHOU Kuo-chih. A new route to produce submicron Mo powders via carbothermal pre-reduction followed by deep magnesium reduction [J]. JOM, 2018, 70: 2561-2566.
[20] SCHUBERT W D, LASSNER E. Production and characterization of hydrogen-reduced submicron tungsten powders-Part 1: State of the art in research, production and characterization of raw materials and tungsten powders [J]. International Journal of Refractory Metals and Hard Materials, 1991, 10: 133-141.
[21] ZIMMERL T, SCHUBERT W D, BICHERL A, BOCK A. Hydrogen reduction of tungsten oxides: Alkali additions, their effect on the metal nucleation process and potassium bronzes under equilibrium conditions [J]. International Journal of Refractory Metals and Hard Materials, 2017, 62: 87-96.
[22] WANG Da-hang, SUN Guo-dong, ZHANG Guo-hua. Preparation of ultrafine Mo powders via carbothermic pre-reduction of molybdenum oxide and deep reduction by hydrogen [J]. International Journal of Refractory Metals and Hard Materials, 2018, 75: 70-77.
[23] MEDEIROS F F P, de OLIVEIRA S A, de SOUZA C P, de SOUZA J F. Synthesis of tungsten carbide through gas-solid reaction at low temperatures [J]. Materials Science and Engineering A, 2001, 315: 58-62.
高纯超细钨和碳化钨粉体的制备
王凯飞,张国华
北京科技大学 钢铁冶金新技术国家重点实验室,北京 100083
摘 要:提出一种两步法制备高纯度W或WC粉体的工艺,该工艺包括碳热预还原WO2.9,以及后续的对还原产物进行H2还原或CH4+H2混合气体碳化。研究C/WO2.9摩尔比和反应温度对不同阶段产物的相组成、形貌、粒径及杂质含量的影响。结果表明,当C/WO2.9摩尔比为2.1:1-2.5:1时,碳热预还原产物的物相由W和少量WO2组成。随着C/WO2.9摩尔比从2.1:1增加到2.5:1,碳热预还原产物的粒径逐渐减小。因此,采用相对较高的C/WO2.9摩尔比和较低的反应温度有利于获得超细W或WC粉体。经过第二阶段的反应,可以制备高纯度W粉和WC粉。
关键词:碳热预还原;渗碳反应;钨;碳化钨
(Edited by Bing YANG)
Foundation item: Project (51725401) supported by the National Natural Science Foundation of China
Corresponding author: Guo-hua ZHANG; Tel: +86-10-82377750; E-mail: ghzhang0914@ustb.edu.cn
DOI: 10.1016/S1003-6326(20)65331-6
Abstract: High-purity ultrafine W or WC powder was prepared via a two-step process composed of the carbothermic pre-reduction of WO2.9 and the following deep reduction with H2 or carbonization with CH4+H2 mixed gases. The effects of C/WO2.9 molar ratio and temperature on phase composition, morphology, particle size, and impurity content of products were investigated. The results revealed that when the C/WO2.9 ratio was in the range from 2.1:1 to 2.5:1, the carbothermic pre-reduction products consisted of W and a small amount of WO2. With changing C/WO2.9 ratio from 2.1:1 to 2.5:1, the particle sizes were gradually decreased. In order to prepare ultrafine W or WC powder, a relatively high C/WO2.9 ratio and a lower reaction temperature at this stage were preferred. After the second reaction, the final products of ultrafine W and WC powders with a high purity could be obtained, respectively.


