DOI:10.19476/j.ysxb.1004.0609.2019.11.02
HBN粒径对7075-T6铝合金PEO膜结构和性能的影响
王琪超1,王帅星1,杜 楠1,程法嵩2,赵 晴1,李新义3
(1. 南昌航空大学 轻合金加工科学与技术国防重点学科实验室,南昌 330063;
2. 中国航发贵州黎阳航空动力有限公司,黎阳 550014;
3. 北京科技大学 腐蚀与防护中心,北京 110083)
摘 要:
摘 要:在硅酸盐和磷酸盐混合电解液体系中加入不同粒径HBN颗粒,通过等离子体氧化法(PEO)在7075-T6合金表面制备Al2O3/HBN复合膜;利用SEM、EDS、XRD、磨损实验、电化学极化曲线等对复合膜的组织结构、化学组成、耐磨及耐蚀性能进行表征。结果表明:HBN颗粒加速了膜层的击穿放电过程,对PEO膜的前期生长有促进作用,且所得膜层较为致密,但膜层粗糙度有所增加。电解液中加入4g/L的HBN颗粒,无论HBN粒径为5、10或30 μm,均能使PEO膜的表层微孔数量及内部缺陷有所减少,且复合膜中晶态Al2O3相含量也有所增长。相比于PEO膜,HBN颗粒复合的PEO/HBN复合膜的摩擦因数更小、比磨损率更低、摩擦磨损性能均有所改善;且HBN粒径为10 μm时,复合膜的耐磨性更佳,比磨损率降低近42.5%。此外,PEO/HBN复合膜的耐蚀性更优,其自腐蚀电流密度较未复合的PEO膜下降了1个数量级;5 μm粒径HBN复合的膜层经模拟海水浸泡1800 h后表面腐蚀较轻微。
关键词:
文章编号:1004-0609(2019)-11-2459-12 中图分类号:TG174.44 文献标志码:A
7075铝合金因其密度小、比强度高、力学性能优异而被广泛应用于交通运输、航空航天及军事国防工业[1]。然而,对铝合金来说获得高的强度和好的耐蚀性一般是矛盾的;T6峰值时效处理虽可获得高的静强度,但在腐蚀介质作用下易发生晶间腐蚀、剥蚀、应力腐蚀及腐蚀疲劳等腐蚀破坏[2-3];并且铝合金的硬度相对较低,在干摩擦条件下往往发生粘着或磨粒磨损[4],导致其耐磨性较差。因此,通常需采用表面处理来提高7075-T6铝合金的防护性能。目前,阳极氧化[5],磁控溅射[6-7]、喷涂[8-9]、离子注入[10]等是常用的铝合金表面处理手段。
近年来,等离子体氧化(PEO),又称微弧氧化(MAO),作为前沿、环保的表面处理技术受到越来越多地关注;该技术可利用电化学、热化学、等离子化学等效应在Al、Mg、Ti等合金表面原位生长出以基体氧化物为主、辅以电解液组分的陶瓷层[11-14],从而满足耐腐蚀、抗磨损、电绝缘等多种需求[15-18]。研究表明,微弧氧化处理的7075铝合金的表面硬度可达1500HV,耐磨性相比于基体的可提高3~5倍,耐中性盐雾试验时间在2000 h以上[17-18]。然而,微弧氧化膜表面的多孔形态会导致涂层摩擦因数不稳定、后期磨损加剧[18-19],且微孔会成为腐蚀介质提供迁移通道、损伤耐蚀性。
目前,已有学者通过颗粒复合PEO技术来进一步提高铝合金PEO膜的耐蚀/耐磨性能,并研究了颗粒增强体对PEO膜形成过程及膜层结构的影响[20-25]。MASOUD等[20]发现在7075铝合金PEO膜中复合适量α-Al2O3颗粒能有效改善其孔隙率,从而提高膜层的耐蚀耐磨性能;VAGANOV-VIL'KINS等[21]利用IR谱等手段表征了PTFE颗粒在5056铝合金表面PEO膜的分布情况,对复合膜的力学性能、耐蚀性和热行为进行了验证;EHSAN等[22]通过电化学诱导PEO膜掺杂TiO2颗粒进一步提高7075铝合金的耐蚀、耐磨性能。此外,诸多学者也将ZrO2、SiC、Cr2O3等微粒引入到镁合金、钛合金微弧氧化膜中,制备出性能更优的PEO复合膜[23-25]。
六方BN微粒(HBN),具有类石墨结构及良好的高温稳定性,片状的BN可以起到良好的减摩作用;目前,已有报道将BN颗粒加入PEO膜中改善其耐磨性[26-27]。然而,等离子体氧化涉及氧化膜成膜、击穿、熔化、凝固、再熔化等一系列过程[28-29],等离子放电的高温高压、放电火花的持续时间均会对第二相颗粒的复合产生影响;郝千驹等[30]发现钛合金复合PEO过程中,较小尺寸(50 nm)的W颗粒溶解消失,较大尺寸(>1 μm)的W颗粒仅发生了表层溶解。因此,为了保证PEO/HBN复合膜的性能,有必要系统研究HBN颗粒尺寸对7075-T6铝合金PEO膜生长过程、表面特征、膜结构及成分和性能的影响。
1 实验
实验材料为7075-T6铝合金,其主要化学成分(质量分数)为5.8% Zn、2.6% Mg、1.5% Cu、0.5% Fe、0.4% Si、0.3% Mn、0.2% Cr、0.2% Ti、余量Al。试样尺寸20 mm×20 mm×3 mm;试样经320~1200# SiC砂纸依次打磨,丙酮超声清洗20min后自然风吹干备用。等离子体氧化前,试样用20 g/L NaOH + 30 g/L Na2CO3 +0.5g/L C12H25SO4Na混合液碱蚀除油,再经50% HNO3和10% HF的混合出光液进行表面出光。
PEO实验使用哈工大WHD-20型微弧氧化电源进行,电参数如下:电流密度15 A/dm2、频率500 Hz、占空比50%、双极性直流脉冲输出方式、氧化时间40 min。氧化中,试样作阳极,70 mm×70 mm×5 mm不锈钢板作阴极,两极间距为120 mm。电解槽使用循环水冷玻璃槽,实验过程通过DC-0510型低温冷却机使等离子体氧化过程始终保持在15 ℃以下进行。电解液采用硅酸盐和磷酸盐混合电解液,并添加不同含量及不同粒径的HBN颗粒。其中,HBN颗粒的平均粒径分别为5、10和30 μm,其粒径分布如图1所示;颗粒均经碱蚀液碱洗后在电解液中进行超声分散,HBN的添加浓度分别为2、4和6 g/L,氧化过程全程机械搅拌。
氧化后,使用Elcometer456涡流测厚仪测量氧化膜膜厚,取10组样点平均值;利用sartorius CPA225D型电子天平(精度0.01 mg)和JB-6C型粗糙度轮廓仪分别测量试样PEO前后质量和表面粗糙度。借助Nova Nano SEM450型场发射扫描电镜观察膜层表面和截面形貌,利用INCA X-Max50型能谱仪分析表面和截面元素分布;通过Bruker D8-Advance A25型X射线衍射仪分析膜层相成分,Cu靶,Kα射线,加速电压40 kV,电流40 mA,步长0.02°,扫描区间10°~ 80°。经CFT-I型摩擦磨损试验机进行摩擦磨损实验,摩擦副为d 5 mm的Si3N4球(HV2200),转速600 r/min,载荷2 N,摩擦半径3 mm,试验时间15 min;磨损试验后,采用KH-7700型三维视频显微镜观察磨痕形貌,拟合磨痕剖面面积,计算比磨损率;比磨损率计算公式如式(1)所示,其中r为磨痕半径,S为磨痕截面面积,N、R分别为载荷和滑动距离,单位mm3/(N·m)。利用CHI604D型电化学工作站检测试样的动电位极化(Tafel)曲线,扫描范围-1.0~-0.5 V (vs. SCE),扫描速率5 mV/s,测量前试样经3.5%NaCl水溶液浸泡0.5 h;同时,通过1800 h的 Mocledon模拟海水浸泡实验评价复合膜的耐蚀性,用普通光学相机和KH-7700型三维视频显微镜观察试样表面的腐蚀形貌。
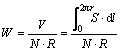 (1)
(1)
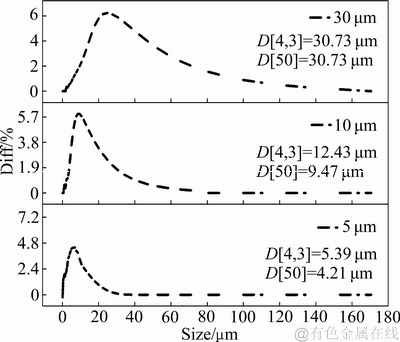
图1 不同尺寸HBN颗粒粒径分布曲线
Fig. 1 Distribution curves of HBN particle with different sizes
2 结果与分析
2.1 PEO复合膜制备
图2和3所示分别为复合膜制备过程中槽电压变化曲线和PEO膜厚、粗糙度变化曲线。氧化过程被分为3个阶段[28-29],分别为阳极氧化、火花放电和弧光放电阶段。阳极氧化膜的形成是PEO过程的必要条件并且氧化膜临界击穿电压取决于金属自身特性和电解液性质[28, 31]。阳极氧化阶段成膜较为致密,击穿电压可由膜厚主导,即膜生长到达固定厚度便可击穿,因此击穿电压可视为固定值。图2和图3(a)所示,电解液中添加定量HBN颗粒,PEO过程膜表面存在的游离HBN颗粒改变膜液界面电场分布,使膜厚无需达到固定值便可形成足够引导击穿的膜电阻,从而加速膜层击穿。均匀击穿阶段被拉长导致前期膜层生长加快,该行为主要体现在阳极氧化阶段和火花放电阶段的槽电压变化,终止电压并未发生明显变化。
此外,PEO膜表面颗粒沉积,必会使表面粗糙度有所增大,其中复合颗粒粒径较小时更易被吸附至膜表面,导致粗糙度增量更大,如图3(b)所示。假设PEO膜成分均为Al2O3且均匀分布,HBN对膜质量影响可忽略,可将致密度计算公式视作如式(2)所示:
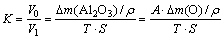 (2)
(2)
式中:A为与摩尔质量相关的常数;T、S分别表示膜厚和PEO暴露面积;ρ为PEO膜密度。
经转化得式(3),试样质量增量与膜厚近似成正比,斜率可侧面反映致密度K (B为常数)。
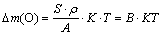 (3)
(3)
图4所示为试样质量增量随氧化膜厚度的变化情况及其线性拟合,分析可知,加入不同尺寸的HBN颗粒后BK值均有不同程度的提升,可以看出膜层致密度有所改善。综上,电解液体系中分别加入2~6 g/L的5、10、30 μm HBN颗粒均会加速膜层击穿,且在4 g/L HBN颗粒复合下氧化时间在50 min内能有效促使膜厚增长,致密度也有明显改善,只有5 μm HBN颗粒复合时粗糙度增量稍大。
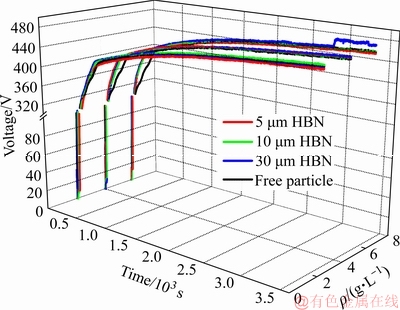
图2 不同尺寸和浓度HBN颗粒复合的PEO过程槽电压 曲线
Fig. 2 Cell voltage curves in PEO process in electrolytes with HBN particles of different sizes and concentrations
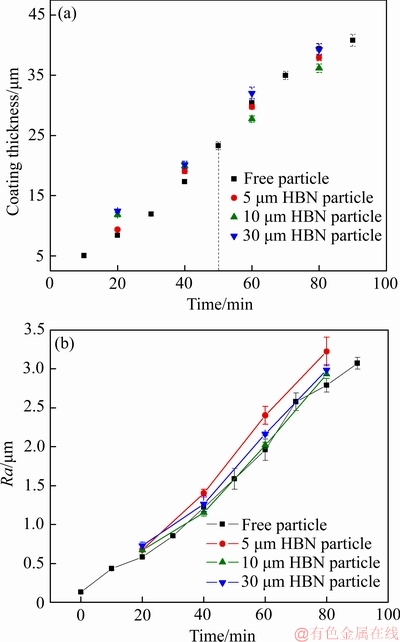
图3 4 g/L浓度下不同尺寸HBN颗粒复合的PEO膜生长曲线和粗糙度变化曲线
Fig. 3 Growth curves (a) and surface roughness curves (b) of PEO coatings formed in electrolytes containing 4 g/L HBN particles with different sizes
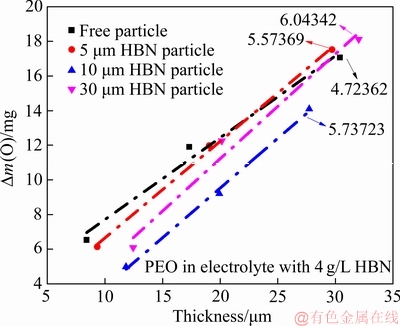
图4 PEO复合膜质量增量随膜厚的变化情况及线性拟合
Fig. 4 Variation law and linear fit curve of mass increment of PEO coatings with different thicknesses
2.2 复合膜微观结构及元素分布
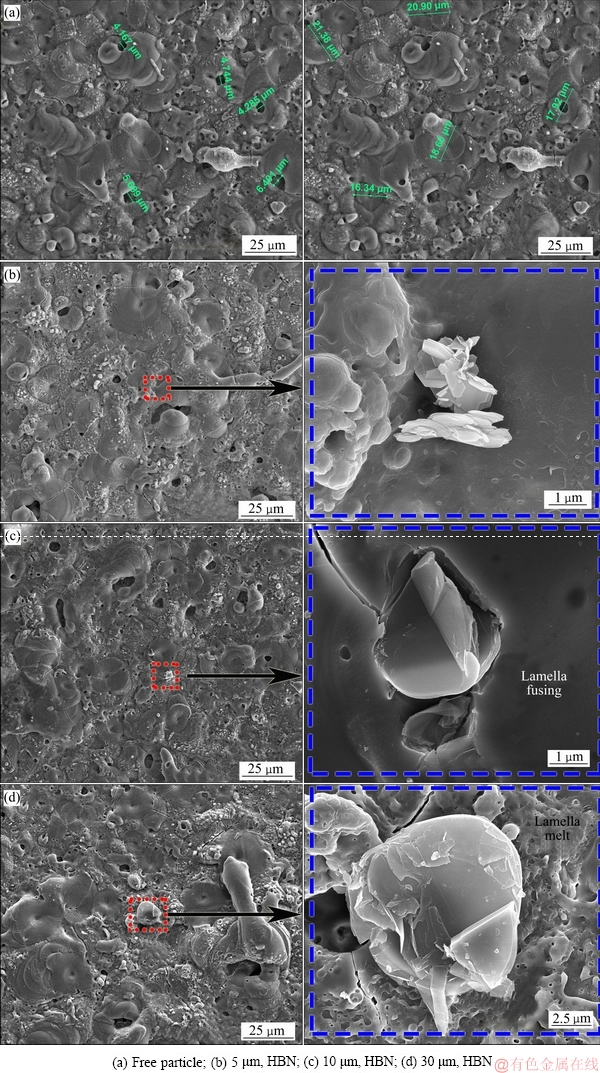
图5 不同尺寸HBN颗粒复合制备的PEO膜表面SEM形貌
Fig. 5 Surface SEM images of PEO coatings formed in electrolytes with HBN particles of different sizes
图5所示为PEO复合膜的表面SEM形貌。如图5(a)所示,基础电解液中制备的PEO膜表面存在较均匀分布的微孔,且孔径多在4~6 μm之间;也能观察到数量较多、分布较均匀的饼状等离子体放电口,其尺寸在17~21 μm左右,饼状物间熔融物并不连续, 有叠加放电迹象。电解液中加入4 g/L不同尺寸的HBN颗粒后,所得的PEO复合膜表面微观形貌均有所改善,4 μm以下的孔洞数量减少,饼状物数量减少;并且得益于放电过程的延长,其间由于更多熔融物的喷射、凝固、烧结,沟壑过渡区也有所减缓。此外,由局部放大SEM像可以发现,片层状HBN颗粒主要以物理沉积方式分布于表面;因报道称等离子体放电温度高达3000~10000 K[28-32],远高于HBN熔点,大量颗粒在放电口瞬间分解,所以能寻获的外表面附着HBN颗粒较少;利用较大粒径BN颗粒复合时发现片层状结构在PEO过程易被熔断和部分熔融,图5(c)和(d)局部放大图发现片层有明显的熔融迹象。
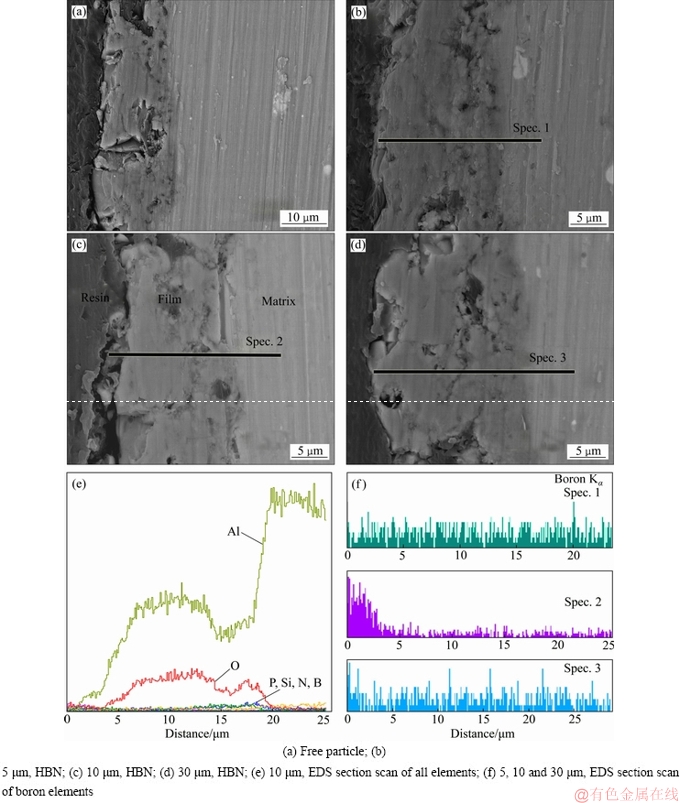
图6 不同尺寸HBN颗粒复合制备的PEO膜的截面SEM形貌及EDS线扫能谱
Fig. 6 Section SEM images of PEO coatings formed in electrolytes with HBN particles of different sizes((a)-(d)) and EDS scan spectrum of all elements (e) and B element (f) of PEO coatings formed in electrolyte with 10 μm HBN particle
图6(a)~(d)所示为不同PEO复合膜截面SEM形貌;图6(e)所示为10 μm HBN颗粒复合下PEO膜的截面EDS线扫谱,图6(f)所示为不同粒径HBN颗粒复合时PEO膜中B元素沿截面分布曲线。如图6(a)~(d)所示,基础电解液中制备的PEO膜内部存在较大的裂纹和孔隙,且这些缺陷几乎贯穿整个膜层;复合5、10和30 μm HBN颗粒后,由于加速击穿导致火花放电阶段的延长,内部氧化物不断重熔烧结,内部孔隙不断被其填充,导致膜厚和致密度均有所增加。通过对各复合膜的截面EDS线扫描发现,Si、P、Al、O元素分布较未复合的膜层无显著变化,5 μm和30 μm HBN颗粒复合的PEO膜截面几乎无法检测到BN颗粒,10 μm HBN颗粒复合下才在膜层近表面检测到了少量的B元素,如图6(e)~(f)所示。因此,BN颗粒除了在膜表面进行物理沉积以外,适中粒径的颗粒(约10 μm)因短时高温发生表层溶解,且被熔融氧化物包裹于膜层近表面层;尺寸较小的BN颗粒(≤5 μm)由于较高的击穿温度完全发生熔融,故而此涂层中B元素无明显聚集,如图6(f)中Spec.1所示;而较大的BN颗粒(≥30 μm)虽存在复合的可能,但受膜厚限制,氧化物不能完全包裹BN颗粒,因此该涂层内部几乎无B元素存在,如图6(f)中Spec.3所示。
综上可知,无论HBN颗粒尺寸多大,当电解液中加入4 g/L HBN颗粒时,PEO复合膜的表面微观形貌均有所改善,内部孔隙较未复合的膜层有所减少。
2.3 复合膜的相组成
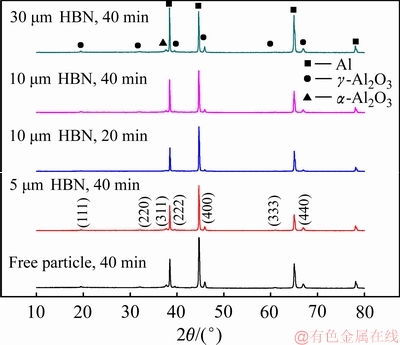
图7 不同HBN颗粒尺寸及氧化时间下PEO复合膜的XRD谱
Fig. 7 XRD patterns of PEO coatings prepared in electrolytes with different HBN particles and different oxidation times
图7所示为不同尺寸HBN颗粒复合下氧化所得的PEO复合膜的XRD谱。PEO膜中检测到了γ-Al2O3 相和少量的α-Al2O3相,且Al2O3晶粒生长具有一定取向;加入一定量的HBN颗粒几乎未明显改变相组成,也未对Al2O3晶粒衍射取向有所影响,但氧化膜中各相含量有所变化,具体表现为γ-Al2O3和α-Al2O3相含量均有所增加。分析可知,由于高温高压作用,只有少量HBN颗粒随熔融氧化物在熔池附近凝固烧结,放电火花口的HBN更是因等离子体放电的超高温被分解,所以XRD谱中并未发现BN相。
结合图5中复合膜表面形貌及图6(e)和(f)中的EDS线扫描结果,可以认为HBN颗粒主要以物理沉积方式附着于PEO膜上,而粒径适中的HBN颗粒还可以未完全熔化的固体形态存在于近表面层中。
2.4 复合膜的耐摩性
图8所示为不同尺寸HBN颗粒复合的PEO膜与Si3N4对磨时的摩擦因数变化曲线及比磨损率,图9所示为其对应的磨损SEM形貌。从图8(a)可知,7075-T6基体因其表面硬度较低,除粘着磨损外还极易发生磨粒磨损[4],磨损过程很不稳定,摩擦因数在0.35~0.5之间波动,比磨损率高达3.89×10-4 mm3/(N·m)。经PEO处理后,7075-T6铝合金的摩擦因数虽有显著增大,但摩擦因数较为稳定、磨损程度明显减轻、比磨损率相对基体降低了约43.7%。当PEO膜层中复合了各尺寸HBN颗粒后,PEO复合膜的减摩效果类似,摩擦因数较未复合的膜层下降了0.15~0.2左右,且摩擦因数更为稳定、磨损程度更轻微。图8(b)显示,未复合、复合5 μm HBN、10 μm HBN及30 μm HBN颗粒的PEO膜的比磨损率分别为2.19×10-4、1.52×10-4、1.26×10-4、1.60×10-4 mm3/(N·m);相同条件下,PEO复合膜的比磨损率均有所减小;且只有HBN颗粒尺寸适中,PEO复合膜的耐磨性才更佳,10 μm HBN颗粒复合时PEO膜的比磨损率相对最低。
由图9(a)可以看出,未复合的PEO膜层表面磨痕极不均匀并存在明显的磨粒磨损特征,由于对磨时的剪切力较大的,形成的犁沟两侧存在较大的团状突起物,显著影响磨损过程[18, 20]。当PEO膜层中复合了各尺寸HBN颗粒后,表面磨痕较为均匀,表面可见粘着磨损特征,但并未出现磨粒磨损形成的犁沟;并且对于10 μm HBN颗粒复合的PEO膜层,表面粘着也较轻微,主要以疲劳磨损形成的“鳞状”剥落和少许微裂纹为主。对于PEO膜层的磨损过程,一般认为是由膜层表面存在的微凸体作为粗糙峰,与另一摩擦副形成峰间的剪切,形成粘着磨损和疲劳磨损特征[18-20];随后大量软粗糙峰被磨平后,产生大量磨削,磨损时易进入峰间较软面,形成磨粒磨损的同时产生大量犁沟,部分硬粗糙峰与软平面间对磨也会产生犁沟。当PEO膜层复合HBN颗粒后,层状HBN之间的分子作用力可以替代对磨副之间的部分作用力,从而减轻载荷作用、降低摩擦因数[27, 33]。当摩擦达到某种程度时,HBN颗粒完整、均匀分布在对磨面上,摩擦处于稳定状态,因而复合膜表面磨损程度明显减轻,耐磨性得到提升。此外,适中的粒径更利于HBN颗粒在膜层表面的物理沉积、提升HBN复合量,进而表现出更佳的抗磨损性能;如图8和9所示,10 μm HBN颗粒复合下PEO膜层的综合耐磨性最好。
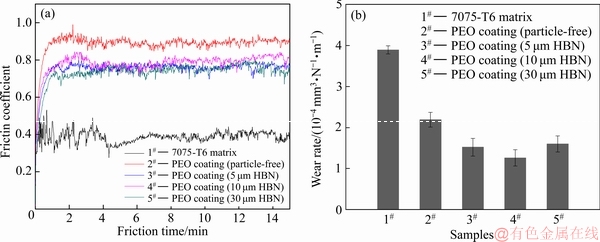
图8 不同尺寸HBN颗粒复合的PEO膜的摩擦因数曲线和比磨损率
Fig. 8 Friction coefficient curves (a) and wear rate (b) of PEO composite coatings formed in electrolytes with HBN particles of different sizes
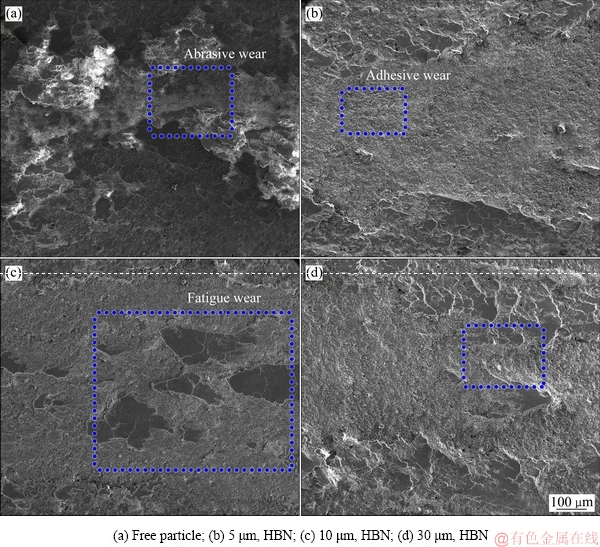
图9 不同尺寸HBN颗粒复合的PEO膜与Si3N4球对磨的磨损SEM像
Fig. 9 SEM images of PEO coatings formed in electrolytes containing different HBN particles after wear testing against with Si3N4
2.5 复合膜的耐蚀性
图10所示为不同尺寸HBN颗粒复合的PEO膜在3.5%NaCl溶液中的Tafel极化曲线,表1所列为对应的拟合结果。由图10和表1可以看出:7075-T6合金基体的自腐蚀电流密度为3.273×10-4 A/cm2左右;经PEO处理后,试样的自腐蚀电位均发生不同程度的正移,腐蚀电流密度大幅降低,即使是未复合的PEO膜层的自腐蚀电流密度相对于7075-T6基体的也降低了2个数量级;这主要是由于PEO膜层的存在阻碍腐蚀介质的迁移,氧化膜起到了屏蔽和钝化效果,抑制了腐蚀反应的发生。此外,当PEO膜层中复合HBN颗粒后,试样的自腐蚀电流较未复合试样的有明显的减小趋势,大约下降了1个数量级。一般认为,PEO膜层的耐蚀性主要受4个因素影响:膜厚,微观结构,物相和缺陷[32, 34];当存在HBN颗粒复合时,除相含量不同外,复合膜的厚度增加,表层孔隙及内部缺陷明显减少,膜层致密度显著改善,这些因素的共同作用增强了膜层的腐蚀阻力,改善了其耐蚀性。对于不同PEO/HBN复合膜,虽然其自腐蚀电流密度较为接近,但相比之下,5 μm HBN颗粒复合的PEO膜层厚度略薄,因而该涂层显示出更为优越的耐腐蚀性能。
此外,通过Mocledon模拟海水浸泡实验进一步对比了PEO膜、PEO/HBN复合膜的耐腐蚀性能,图11所示为不同试样浸泡1800 h后的表面OM形貌。可以明显看出:7075-T6合金表面产生了大量白絮状腐蚀产物,微观表面均分布着大量的黑色腐蚀斑,耐蚀性很差,如图11(a)所示;当7075合金表面覆盖了PEO涂层之后,试样的腐蚀倾向大幅降低;无论复合HBN与否,PEO膜浸泡1800 h后表面都只有少量局部絮状腐蚀斑或者蚀孔,耐蚀性大大优于7075铝合金基体的。然而,不同结构的氧化膜耐蚀性存在一些显著差异。对比图11(b)和(c) 可知,并非氧化时间越长PEO膜层的耐蚀性就好,PEO膜的耐蚀性与膜层结构之间关系更为密切;PEO时间过长时,膜层表面放电微孔孔径增大,Cl-等腐蚀介质更易进入膜层从而接触到基体,加速膜层腐蚀,因而该试样表面的腐蚀程度略重于氧化40 min的试样,表面存在局部网絮状的腐蚀斑。而从图11(b)和(d)可以看出,在相同的氧化时间(40 min)下,HBN颗粒复合的PEO膜层耐蚀性更佳;未复合的PEO膜浸泡1800 h后,表面存在一定数量的黑色蚀孔(见图11(b)),而PEO/HBN复合膜浸泡后表面蚀点数量明显更少(见图11(d)),这也进一步说明致密的膜层结构有助于改善PEO膜层的耐蚀性。
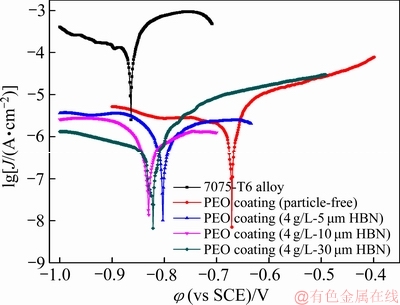
图10 不同尺寸HBN颗粒复合的PEO膜(氧化40 min)的Tafel极化曲线
Fig. 10 Tafel curves of samples processed by PEO in electrolytes with different HBN particles for 40 min
表1 不同试样在3.5%NaCl水溶液中的腐蚀电流密度和自腐蚀电位
Table 1 Corrosion current density and corrosion potential of different samples in 3.5%NaCl solution
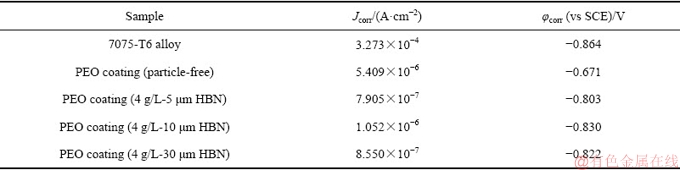
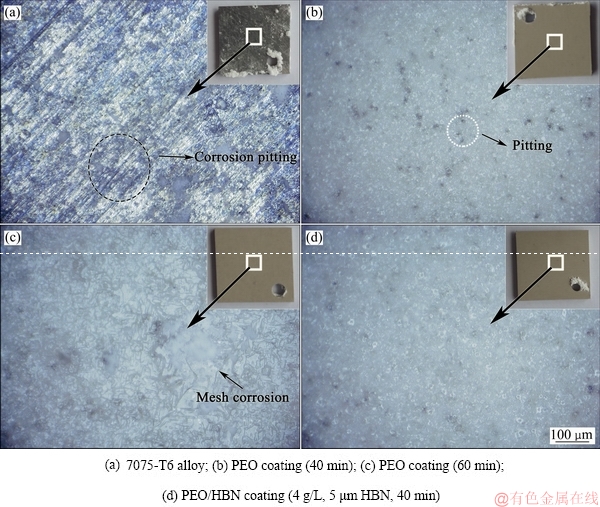
图11 不同试样经Mocledon模拟海水浸泡1800 h的表面腐蚀形貌
Fig. 11 Surface corrosion morphologies of different samplesafter 1800 h Mocledon simulated seawater immersion experiment
3 结论
1) 不同尺寸的HBN颗粒均会不同程度地加速7075铝合金表面PEO膜的击穿放电,延长火花放电阶段,促进PEO膜的前期生长。
2) PEO膜中复合各种尺寸的HBN颗粒均对膜层微观形貌有所改善,膜层表面微孔数量及内部缺陷明显减少,致密度得到提高。HBN颗粒主要以物理沉积方式存在于膜层表面及近表层;HBN颗粒的复合几乎未改变PEO膜的相组成,但复合膜中晶态Al2O3相含量有所增长。
3) 相比于PEO膜,所有HBN颗粒复合的PEO/HBN复合膜的摩擦因数、比磨损率均有所降低,摩擦磨损性能均有所改善;且HBN粒径为10 μm时,复合膜的耐磨性更佳,比磨损率降低近42.5%。复合后,表面磨痕较为均匀,磨损特征主要为粘着磨损,还存在少量疲劳磨损迹象。
4) 致密的膜层结构有助于改善PEO膜层耐蚀性;PEO/HBN复合膜的自腐蚀电流密度较未复合的PEO膜下降了1个数量级,且5 μm HBN颗粒复合时所制备的PEO复合膜的自腐蚀电流密度略小、耐蚀性更为优越,经Mocledon模拟海水浸泡1800 h后表面腐蚀较轻微。
REFERENCES
[1] HEINZ A, HASZLER A, KEIDEL C, MOLDENHAUER S, BENEDICTUS R, MILLER W S. Recent development in aluminium alloys for aerospace applications[J].Materials Science & Engineering A,2000, 280(1): 102-107.
[2] HOLROYD N J H, SCAMANS G M. Stress corrosion cracking in Al-Zn-Mg-Cu aluminum alloys in saline environments[J]. Metallurgical and Materials Transactions, 2013, 44(3): 1230-1253.
[3] 刘继华, 李 荻, 刘培英. 热处理对7075铝合金应力腐蚀及断口形貌的影响[J]. 材料热处理学报, 2010, 31(7): 109-113.
LIU Ji-hua, LI Di, LIU Pei-ying. Effect of heat treatment on stress corrosion behavior of 7075 aluminum alloy[J]. Transactions of Materials and Heat Treatment, 2010, 31(7): 109-113.
[4] BAYDOGAN M, CIMENOGLU H, KAYALI E S. A study on sliding wear of a 7075 aluminum alloy[J]. Wear, 2004, 257(7): 852-861.
[5] DU N, WANG S X, ZHAO Q, SHAO Z S. Effects of boric acid on microstructure and corrosion resistance of boric/ sulfuric acid anodic film on 7050 aluminum alloy[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(7): 1655-1660.
[6] DOMINGUEZ-CRESPO M A, RODIL S E, TORRES- HUERTA A M, RAMIREZ-MENESES E, SUAREZ- VELAZQUEZ G. Structural and electrochemical performance of sputtered Al-Ce films on AA6061 aluminum alloy substrates[J]. Surface and Coatings Technology, 2009, 204(5): 571-579.
[7] KISHORE KUMAR P R, MANIKANDAN V N, DEEPAK RAJ P, SRIDHARAN M. Characterization of magnetron sputtered Si3N4 thin films deposited on aluminum alloy substrates[J]. Materials Today: Proceedings, 2016, 3(6): 1536-1540.
[8] NGAI S, NGAI T, VOGEL F, STORY W, THOMPSON G B, BREWER L N. Saltwater corrosion behavior of cold sprayed AA7075 aluminum alloy coatings[J]. Corrosion Science, 2018, 130: 231-240.
[9] BAO Y Q, GAWNE D T, GAO G M, ZHANG T, CUENCA B D, ALBERDI A. Thermal-spray deposition of enamel on aluminium alloys[J]. Surface and Coatings Technology, 2013, 232: 150-158.
[10] 刘洪喜, 王浪平, 王小峰, 汤宝寅. LY12CZ铝合金表面等离子浸没离子注入氮层的摩擦磨损性能研究[J]. 摩擦学学报, 2006(5):417-421.
LIU Hong-xi, WANG Lang-ping, WANG Xiao-feng, TANG Bao-yin. Friction and wear behaviors of 2024 aluminum alloys by nitrogen plasma immersion ion implantation[J]. Tribology, 2006(5): 417-421.
[11] WALSH F C, LOW C T, WOOD R J K, STEVENS K T, ARCHER J, POETON A R. Plasma electrolytic oxidation(PEO) for production of anodised coatings on light weight metal(Al, Mg, Ti) alloys[J]. Transactions of the Institute of Metal Finishing, 2009, 87(3): 122-135.
[12] MATYKINA E, ARRABAL R, SCURR D J, BARON A , SKELDON P, THOMPSON G E. Investigation of the mechanism of plasma electrolytic oxidation of aluminium using 18O tracer[J]. Corrosion Science, 2010, 52(3): 1070-1076.
[13] WEN L, WANG Y M, ZHOU Y, OUYANG J H, GUO L X, JIA D C. Corrosion evaluation of microarc oxidation coatings formed on 2024 aluminium alloy[J]. Corrosion Science, 2010, 52(8): 2687-2696.
[14] JAVIDI M, FADAEE H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior[J]. Applied Surface Science, 2013, 286: 212-219.
[15] DEHNAVI V, LUAN B L, SHOESMITH D W, LIU X Y, ROHANI S. Effect of duty cycle and applied current frequency on plasma electrolytic oxidation (PEO) coating growth behavior[J]. Surface and Coatings Technology, 2013, 226: 100-107.
[16] HAKIMIZAD A, RAEISSI K, SANTAMARIA M, ASGHARI M. Effects of pulse current mode on plasma electrolytic oxidation of 7075 Al in Na2WO4 containing solution: From unipolar to soft-sparking regime[J]. Electrochimica Acta, 2018, 284: 628-629.
[17] VENUGOPAL A, PANDA R, MANWATKAR S, SREEKUMAR K, KRISHNA L R, SUNDARARAJAN G. Effect of micro arc oxidation treatment on localized corrosion behaviour of AA7075 aluminum alloy in 3.5% NaCl solution[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(3): 700-710.
[18] 王艳秋, 王 岳, 陈派明, 邵亚薇, 王福会. 7075铝合金微弧氧化涂层的组织结构与耐蚀耐磨性能[J]. 金属学报, 2011, 47(4): 455-461.
WANG Yan-qiu, WANG Yue, CHEN Pai-ming, SHAO Ya-wei, WANG Fu-hui. Microstruture, corrosion and wear resistance of microarc oxidation coating on Al alloy 7075[J]. Acta Metall Sin, 2011, 47(4): 455-461.
[19] WANG Y M, JIANG B L, GUO L X, LEI T Q. Tribological behavior of microarc oxidation coatings formed on titanium alloys against steel in dry and solid lubrication sliding[J]. . Applied Surface Science, 2006, 252: 2989-2998.
[20] GHAFARIPOOR M, RAEISSI K, SANTAMARIA M, HAKIMIZAD A. The corrosion and tribocorrosion resistance of PEO composite coatings containing α-Al2O3 particles on 7075 Al alloy[J]. Surface and Coatings Technology, 2018, 349: 470-479.
[21] VAGANOV-VIL'KINS A A, RUDNEV V S, PAVLOV A D, SUKHOVERKHOV S V, KOSTIN V I, LUKIYANCHUK I V. IR and Py-GC/MS investigation of composite PTFE/PEO coatings on aluminum[J]. Materials Chemistry and Physics, 2019, 221: 436-446.
[22] AKBARI E, DI FRANCESCO F, CEAOLO P, RAEISSI K, SANTAMARIA M, HAKIMIZAD A. Electrochemically- induced TiO2 incorporation for enhancing corrosion and tribocorrosion resistance of PEO coating on 7075 Al alloy[J]. Corrosion Science, 2018, 143: 314-328.
[23] CHEN Y, YANG Y G, ZHANG W, ZHANG T, WANG F H. Influence of second phase on corrosion performance and formation mechanism of PEO coating on AZ91 Mg alloy[J]. Journal of Alloys and Compounds, 2017, 718: 92-103.
[24] ARRBAL R, MATYKINA E, SKELDON P, THOMPSON G E. Incorporation of zirconia particles into coatings formed on magnesium by plasma electrolytic oxidation[J]. Journal of Materials Science, 2008, 43: 1532-1538.
[25] WANG S X, ZHAO Q, LIU D X, DU N. Microstructure and elevated temperature tribological behavior of TiO2/Al2O3 composite ceramic coating formed by microarc oxidation of Ti6Al4V alloy[J]. Surface and Coatings Technology, 2015, 272: 343-349.
[26] AO N, LIU D X, WANG S X, ZHAO Q, ZHANG X H, ZHANG M M. Microstructure and tribological behavior of a TiO2/hBN composite ceramic coating formed via micro-arc oxidation of Ti-6Al-4V alloy[J]. Journal of Materials Science & Technology, 2016, 32: 1071-1076.
[27] 王玉洁, 张 鹏, 杜云慧, 李领雷, 李楠楠, 李 强. LY12铝合金微弧氧化自润滑复合膜的制备与摩擦性能[J]. 中国有色金属学报, 2018, 28(9): 1730-1736.
WANG Yu-jie, ZHANG Peng, DU Yun-hui, LI Ling-lei, LI Nan-nan, LI Qiang. Preparation and tribological properties of micro-arc oxidation self-lubricating composite coatings on LY12 aluminum alloy[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(9): 1730-1736.
[28] DULEAV C S, GOLOSNOY I O, CURRAN J A, CLYNE T W. Characterization of discharge events during plasma electrolytic oxidation[J]. Surface and Coatings Technology, 2009, 203: 3410-3419.
[29] 潘明强, 狄士春. 微弧氧化火花放电等离子体源的形成机制研究[J]. 稀有金属材料与工程, 2011, 40(S2): 333-338.
PAN Ming-qiang, DI Shi-chun. Formation mechanism of the spark discharge plasma source about micro-arc oxidation[J]. Rare Metal Materials and Engineering, 2011, 40(S2): 333-338.
[30] 郝千驹, 杜 楠, 赵 晴, 王帅星, 李新义, 董超芳. TC4钛合金微弧氧化膜形成过程中熔池的温度探测[J]. 表面技术, 2018, 47(4): 51-57.
HAO Qian-ju, DU Nan, ZHAO Qing, WANG Shuai-xing, LI Xin-yi, DONG Chao-fang. Temperature detection of molten bath during formation of micro arc oxidation film on TC4 titanium alloy[J]. Surface Technology, 2018, 47(4): 51-57.
[31] KRYSMANN W, KURZE P, DITTRICH K-H, SCHNEIDER H G. Process characteristics and parameters of Anodic Oxidation by spark discharge (ANOF)[J]. Crystal Research and Technology, 1984(7): 973-979.
[32] SALIH D, METIN U. Characterization and mechanical properties of coatings on magnesium by micro arc oxidation[J]. Applied Surface Science, 2012, 261: 774-782.
[33] PODGORNIK B, KOSEC T, KPCIJAN A, DONIK C. Tribological behaviour and lubrication performance of hexagonal boron nitride (h-BN) as a replacement for graphite in aluminium forming[J]. Tribol Int, 2015, 81: 267-275.
[34] 董海荣, 马 颖, 郭惠霞, 张玉福,郝 远. AZ91D镁合金微弧氧化膜的致密性及其对耐蚀性的影响[J]. 中国有色金属学报, 2015, 25(4): 844-851.
DONG Hai-rong, MA Ying, GUO Hui-xia, ZHANG Yu-fu, HAO Yuan. Compactness of micro-arc oxidation coatings on AZ91D magnesium alloys and its effect on coating corrosion resistance[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(4): 844-851.
Effects of HBN particle sizes on microstructure and performances of PEO coatings on 7075-T6 aluminum alloy
WANG Qi-chao1, WANG Shuai-xing1, DU Nan1, CHENG Fa-song2, ZHAO Qing1, LI Xin-yi3
(1. National Defense Key Discipline Laboratory of Light Alloy Processing Science and Technology, Nanchang Hangkong University, Nanchang 330063, China;
2. AECC Guizhou Liyang Aviation Power Co., Ltd., Liyang 550014, China;
3. Corrosion and Protection Center, University of Science and Technology Beijing, Beijing 100083, China)
Abstract: Al2O3 /HBN composite coatings were prepared on 7075-T6 aluminum alloy by plasma electrolysis oxidation (PEO) in silicate-phosphate mixed electrolyte with HBN particles of different sizes. The microstructure, composition, wear resistance and corrosion resistance of the composite coating were analyzed by SEM, EDS, XRD, wear test and polarization curves. The results show that HBN particles can accelerate the breakdown discharge process of coating, and promote the pre-growth stage of PEO coating. The incorporation of HBN particles also makes PEO coating more dense, but increase the roughness. Whether HBN particle size is 5, 10 or 30 μm, the addition of 4 g/L HBN particles in the electrolyte can reduce the surface micropores number and the internal defects of PEO coating, and make the coating surface be flatter. The content of Al2O3 crystal phase in the PEO composite coatings also increases. Compared with PEO coating, the PEO/HBN composite coatings have smaller friction coefficient, lower wear rate and better wear properties. Among them, the PEO composite coating prepared in electrolyte containing 10 μm HBN particles shows the best wear resistance, and the specific wear rate decreases by 42.5%. Besides, the PEO/HBN composite coatings have better corrosion resistance, the corrosion current density is one order of magnitude lower than that of the uncomposited PEO coating, and the surface corrosion of PEO sample prepared in electrolyte with 5 μm HBN particles is the slightest after 1800 h simulated seawater immersion test.
Key words: 7075-T6 alloy; PEO; HBN particles; micro-structure; performances
Foundation item: Projects(51801094, 51361025) supported by the National Natural Science Foundation of China; Project(20171BAB216006) supported by the Natural Science Foundation of Jiangxi Province, China; Project(GJJ160712) supported by Science and Technology Project of Education Ministry of Jiangxi Province, China
Received date: 2018-11-12; Accepted date: 2019-03-11
Corresponding author: WANG Shuai-xing; Tel: +86-791-86453250; E-mail: wsxxpg@126.com
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51801094,51361025);江西省自然科学基金资助项目(20171BAB216006);江西省教育厅科学技术项目(GJJ160712)
收稿日期:2018-11-12;修订日期:2019-03-11
通信作者:王帅星,讲师,博士;电话:0791-86453250;E-mail:wsxxpg@126.com
[3] 刘继华, 李 荻, 刘培英. 热处理对7075铝合金应力腐蚀及断口形貌的影响[J]. 材料热处理学报, 2010, 31(7): 109-113.
[10] 刘洪喜, 王浪平, 王小峰, 汤宝寅. LY12CZ铝合金表面等离子浸没离子注入氮层的摩擦磨损性能研究[J]. 摩擦学学报, 2006(5):417-421.
[18] 王艳秋, 王 岳, 陈派明, 邵亚薇, 王福会. 7075铝合金微弧氧化涂层的组织结构与耐蚀耐磨性能[J]. 金属学报, 2011, 47(4): 455-461.
[27] 王玉洁, 张 鹏, 杜云慧, 李领雷, 李楠楠, 李 强. LY12铝合金微弧氧化自润滑复合膜的制备与摩擦性能[J]. 中国有色金属学报, 2018, 28(9): 1730-1736.
[29] 潘明强, 狄士春. 微弧氧化火花放电等离子体源的形成机制研究[J]. 稀有金属材料与工程, 2011, 40(S2): 333-338.
[30] 郝千驹, 杜 楠, 赵 晴, 王帅星, 李新义, 董超芳. TC4钛合金微弧氧化膜形成过程中熔池的温度探测[J]. 表面技术, 2018, 47(4): 51-57.
[34] 董海荣, 马 颖, 郭惠霞, 张玉福,郝 远. AZ91D镁合金微弧氧化膜的致密性及其对耐蚀性的影响[J]. 中国有色金属学报, 2015, 25(4): 844-851.


