Synthesis of LiFePO4/C composite electrode with enhanced electrochemical performance
HU Guo-rong(胡国荣), GAO Xu-guang(高旭光), PENG Zhong-dong(彭忠东),
CHEN Zhao-yong(陈召勇), TAN Xian-yan(谭显艳), YU Xiao-yuan(禹筱元)
(School of Metallurgy Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
LiFePO4/C composite was synthesized by high temperature solid-state reaction using iron(Ⅱ) oxalate, ammonium di-hydrogen phosphate and lithium carbonate with a kind of carbohydrate dissolved in the dispersant(ethanol) as carbon sources added to the synthetic precursor. The samples were characterized by X-ray diffraction(XRD), scanning electron microscopy observations(SEM), charge/discharge test, cyclic voltammetry(CV) and carbon analysis. The results show that the synthesis of LiFePO4/C has ordered olivine structure. The carbon has two advantages: optimizing particle size of LiFePO4 and increasing the electronic conductivity and high Li+ diffusivity. The cathode material can demonstrate a charge/discharge flat voltage of 3.4V(vs Li+/Li). Especially the active material with 15% and 20% carbohydrate added according to the final product of lithium iron phosphate shows very good electrochemical performance delivering about initial 150.2mA·h·g-1 and 162.0mA·h·g-1 specific capacity respectively at 0.1C rate and the carbon contents in the final production are only 5.17% and 5.29%, respectively.
Key words:
Li-ion battery; cathode materials; LiFePO4; carbon-coated CLC number: TM911.1;
Document code: A
1 INTRODUCTION
The ever-growing demand for portable batteries with high energy density is exerting pressure for the development of advanced lithium-ion batteries. Commercial Li-ion batteries rely on the application of one of the well-known lithium insertion hosts, i.e. LiCoO2, LiMn2O4 and LiNiO2. However the high cost of cobalt resource, low specific capacity of LiMn2O4 and LiNiO2 is known to be difficult to synthesize and its multi-phase reaction during electrochemical cycling leads to structural degradation. For large-scale applications such as electric and hybrid vehicle systems, the vital issue is the availability of advanced materials. The high temperature performance is also critical because these batteries may be operated at elevated temperature.
LiMPO4 as cathode materials (M=Fe, Mn, FeyMn1-y, Co, Ni, etc.) has attracted much attention as a candidate for the battery with high energy density since Padhi et al[1] established the baseline characteristics of LiFePO4 cathodes for lithium-ion batteries in 1996. LiFePO4 has a high lithium intercalation voltage (3.4V, vs Li /Li+), high theoretical capacity (170mA·h·g-1), excellence in a high temperature application[2], low cost[3], ease of synthesis, and stability when used with common organic electrolyte systems[4-7]. Unfortunately, it should be noted that there is an intrinsic negative aspects of olivine-type materials for lithium battery cathodes—very poor conductivity[1] and Li+ can only be partially extracted/inserted at large charge/discharge rates. About two years ago, Yamada et al[8] and MacNeil et al[9] considered that LiFePO4 reacted very weakly with electrolyte at elevated temperatures compared with other common cathode materials and therefore it may be suitable for large cell applications. In recent optimization of LiFePO4 is focusing on the increase of its electronic conductivity and the synthesis routes[10-12]. In recent years, improvements have been made to improve the conductivity of LiFePO4 through optimization of synthesis techniques to minimize the particle size, doping to improve the intrinsic conductivity[13, 14], addition of metal powder[15]or carbon particles[16, 17] during synthesis or incorporating organic or polymeric additives[18] to form conductive carbon coatings on the particles during firing. In this paper, a study of pure and modified LiFePO4 materials was described. The goal was to prepare active materials with excellent electrochemical performance.
2 EXPERIMENTAL
2.1 Synthesis of cathode materials
The cathode materials were prepared by solid-state reaction of FeC2O4·2H2O(Aldrich), NH4H2PO4 (Aldrich), Li2CO3 (Aldrich) and carbohydrate (Aldrich).The stoichiometric mixtures of the raw materials were mixed by ball-milling (500r/min) for 12h with agate balls in alcohol, followed by drying. Then these mixtures were ground with a mortar and pellets were calcined at 350℃ for 8h in flowing Ar. Final firing for crystallization of the olivine phase was made at 750℃ for 24h in Ar flowing ambience. The samples of L0, L1, L2, L3 and L4 correspond to LiFePO4 with 0%(uncoated), 10%, 15%, 20% and 25% carbohydrate mixed respectively according to the product of LiFePO4.
2.2 Characteristic of cathode materials
2.2.1 Phase identification and particle size analysis
The phase analysis and particle size of all samples were determined by using X-ray diffraction(XRD, D/max-r A type CuKα1140kV, 300mA, 10°-70°, 8°/min, Japan).
2.2.2 Microstructures of cathode material
Scanning electron microscope(SEM) was used to examine the microstructures of the cathode material(SEM, KYKY 2800, Japan).
2.2.3 Electrochemical measurements
The electrochemical cycling performances of the LiFePO4 powders were evaluated at room temperature (20℃) with laboratory-scale Li/LiFePO4 button cells including a lithium metal foil as counter electrode, a composite of 80% LiFePO4, 10% acetylene black (AB) and 10%(mass fraction) polytetrofluornethelene(PTFE) binder as a cathode. A micro-porous polypropylene film (Celgard 2400) was used as a separator and 1mol/L LiPF6 solution with the 1∶1 volume ratio of ethylene carbonate-dimethyl carbonate (EC-DMC) was used as the electrolyte. All cells were assembled inside a glove box filled with ultra-pure argon. Charge/discharge characteristics of the cells were recorded in the potential range of 2.5V and 4.1V using a LAND computer-controlled galvanostat. Currents and specific capacities were calculated based on the mass of LiFePO4 but not LiFePO4/C in the electrode.
The cyclic voltammgram of the powder microelectrode test cell was performed between 2.2V and 4.2V vs lithium using a Potentiostat/Galvanostat system, Model 273A, EG&G instrument. The scan rate was chosen to be 0.5mV/s to 20mV/s. A piece of Pt wire (100μm in diameter) was embedded in glass tubing. Part of the Pt wire was etched by aqua regia, so that a micro-cavity was packed with powders of the cathode material. By using the powder microelectrode as the working electrode, lithium metal foils were chosen as the counter electrode and 1mol/L LiPF6/EC+DMC (1∶1 in volume) as the electrolyte solution, and the test cells were assembled in argon on dry box. As the surface area of the counter electrode was 200 times larger than that of the working microelectrode, the counter electrode could also be used as the reference electrode without serious error.
2.2.4 Carbon concentration
The carbon concentration of the composite was analyzed using CS-444 carbon/sulfur determinator (LECO Co. USA).
3 RESULTS AND DISCUSSION
The phases of all the samples L0, L1, L2, L3 and L4 are shown in Fig.1. From the powder X-ray diffraction (XRD) patters, it can be seen that the synthetic LiFePO4 phase belongs to ordered olivine-type structure. From Table 1, it can be seen that the diffraction intensity of 311 peak of LiFePO4 compound declines with the more carbohydrate mixed in the LiFePO4/C composites. This means that with the increasing amount of carbon coated the amorphous phases in the composite also increase. But their diffraction peaks are in the same positions indicating that carbon coating does not have effect on the inner crystal structure of LiFePO4.
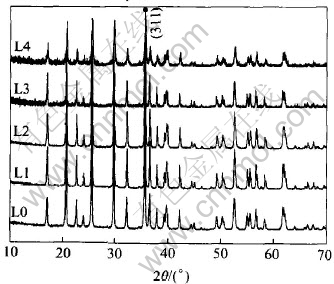
Fig.1 XRD patterns of samples L0, L1, L2, L3 and L4
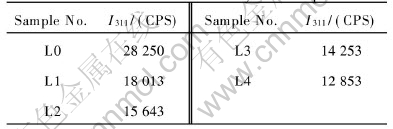
Table 1 Relative intensity of 311 peak
From the SEM photographs of Fig.2, it can also be known that the addition of carbohydrate has an evident effect on controlling the growing particles of cathode powder, and the particle size of LiFePO4/C(L2, L3) is more well-proportioned than that of the pure LiFePO4(L0). The refined LiFePO4 particle coated by carbon can help to increase the diffusivity of lithium ion during the electrode process. A wide size distribution ranging from 0.5μm to 5μm for the LiFePO4 particles can be recognized in Fig.2(a). And the distribution is not uniform, which will make the Li+ of the larger particle not be discharged completely during the electrode process and leads to the low specific capacity of sample L0.
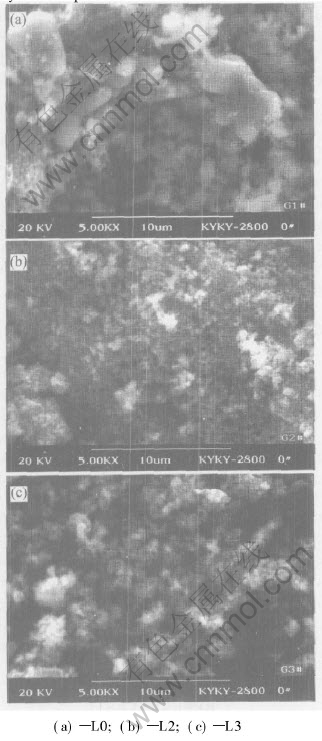
Fig.2 SEM micrographs for samples
The electrochemical insertion/deinsertion of lithium ions into LiFePO4 can be described as the following formula:
Li1-xFePO4=Li1-xFePO4+xLi++xe
This reaction occurs on a flat plateau at 3.4V vs Li, as a two-phase process of FePO4/ LiFePO4, and the complete extraction of lithium(x=1) corresponds to a theoretical specific capacity of 170mA·h·g-1.
From Fig.4, it can be clearly known that the initial capacity decreases with the more than 20% carbohydrate added according to the product of LiFePO4. It can be seen that the pure LiFePO4 (L0) can only keep the specific capacity of 70.5mA·h·g-1 after 20 cycles with the initial specific capacity of 120.4mA·h·g-1 owing to the low electric conductivity[1]. The specific capacity loss is more than 40% while the LiFePO4/C composites show excellent electrochemical behavior. Sample L3 can retain 156.90mA·h·g-1 after 20 cycles with the initial capacity of 162.0mA·h·g-1 and the carbon mass fraction is only 5.29% (Table 2). The capacity loss is very small. The reason is that the carbohydrate as the carbon precursor can improve the active material conductivity and the Li+ diffusivity.
The influence of the scan rate on electrochemical performance of sample L0 and sample L3 was investigated by using power micro-electrode cycle voltammetry. The pair of peaks, consisting of an anodic and a cathodic peak, observed around 3.4V vs Li/Li+ corresponded to the two-phase charge/
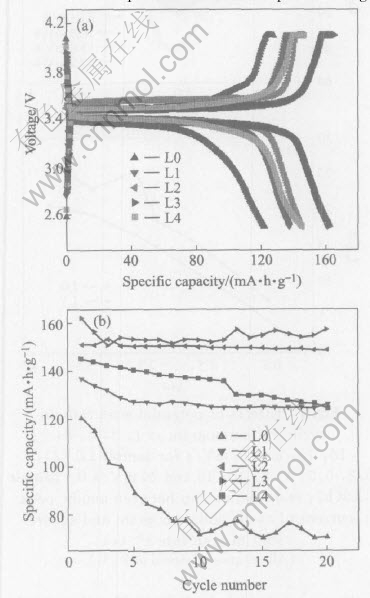
Fig.3 Initial charge-discharge(a) and cycling behaviors(b) profiles of samples L0, L1, L2, L3 and L4
Table 2 Carbon content in samples L2 and L3

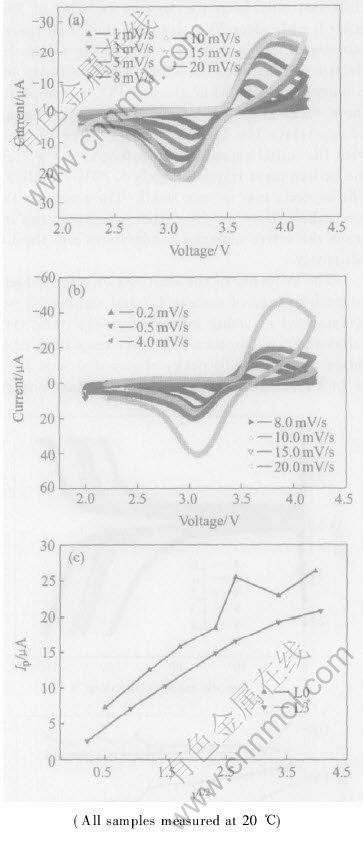
Fig.4 Effects of potential scan rate on cyclic voltammogram at 1, 3, 5, 8, 10, 15, and 20mV/s for sample L0 (a); 0.2, 0.5, 4, 8, 10, 15 and 20mV/s for sample L3(b); and relationship between anodic peak current of cyclic voltammogram and square root of scan rate v0.5(c)
discharge reaction of the Fe2+/Fe3+ redox couple. This voltammogram indicates only one electrochemical reaction. Figs.4(a) and (b) show the effect of the potential scanning rate on the cycle voltammograms measured at 1.0, 3.0, 5.0, 8.0, 10.0, 15.0 and 20.0mV/s for sample L0 and 0.2, 0.5, 4.0, 8.0, 10.0, 15.0 and 20.0mv/s for sample L3 respectively. Although the wave shape of the anodic and cathodic peak is almost symmetrical, the potential separation between the two peaks increases as the scanning rate increases. This result means that the Fe2+/Fe3+ redox reaction is a quasi-reversible process. Fig.4(c) shows the relationship between the peak current and the square root of the scan rate(v0.5). The peak current is proportional to v0.5 with the scan rate is less than about 10mV/s. This means that the electrochemical process is limited by lithium diffusion. When the scan rate exceeds 10mV/s the relationship between Ip and v0.5 is not proportional. This indicates that the electrochemical process is controlled by a similar blend process[19].
While with the increase of the carbon content the volumetric energy density and tap density decrease unfortunately[20]. Huang et al[21] made LiFePO4/C composite by mixing raw materials with carbon gel before heating. In this composite, LiFePO4 has a particle size of 100-200nm and there is about 15% carbon. Consequently the volumetric energy density decreases greatly.
4 CONCLUSIONS
The adding carbohydrate to the synthetic precursor can help to improve the electrical conductivity of LiFePO4 and prevent growth of the particle. The most important thing is that the production of LiFePO4 will be covered by refined carbon uniformity after the precursor has been sintered. It contributes to the transfer of electron and Li+ during the electrode process. Meanwhile the existing carbon can prevent the production of Fe3+ phase to some extent. Sample L3 shows excellent capacity and cycle capability exhibiting about 95% theoretic capacity, 162.0mA·h·g-1, at room temperature. This indicates that LiFePO4/C composite will be an attractive candidate as cathode material for Li-ion battery.
REFERENCES
[1]Padhi A K, Najundaswamy K S, Goodenough J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc,1997, 144: 1188-1194.
[2]Iltchev N, CHEN Yi-ke. LiFePO4 storage at room and elevated temperatures [J]. J Power Sources, 2003, 119-121: 749-754.
[3]Zaghib K, Striebel K, Guerfi A, et al. LiFePO4/polymer/natural graphite: low cost Li-ion batteries [J]. Electrochimica Acta, 2004, 50(2-3): 262-269.
[4]YANG S, SONG Y, Zavalij P Y, et al. Reactivity, stability and electrochemical behavior of lithium iron phosphates [J]. Electrochem Comm, 2002, 4: 239-244.
[5]Jiang J, Dahn J R. ARC studies of the thermal stability of three different cathode materials: LiCoO2; Li[Ni0.1Co0.8Mn0.1]O2; and LiFePO4, in LiPF6 and LiBoB EC/DEC electrolytes [J]. Electrochemistry Communications, 2004, 6(1): 39-43.
[6]Zaghib K, Charest P. Safe Li-ion polymer batteries for HEV applications [J]. J Power Sources, 2004, 134(1): 124-129.
[7]Kim H S, Cho B W, Cho W Ⅱ. Cycling performance of LiFePO4 cathode material for lithium secondary batteries [J]. J Power Sources, 2004, 132(1-2): 235-239.
[8]Yamada A, Chung S C, Hinokuma K. Optimized LiFePO4 for lithium battery cathodes [J]. J Electrochem Soc, 2001, 148: A224-A229.
[9]MacNeil D D, LU Zhong-hua, CHEN Zhao-hui, et al. A comparison of electrode/electrolyte reaction at elevated temperatures for various Li-ion battery cathodes [J]. J Power Sources, 2002, 108: 8-14.
[10]Cho T H, Chung H T. Synthesis of olivine-type LiFePO4 by emulsion-drying method [J]. J Power Sources, 2004, 133(2): 272-276.
[11]Myung S T, Komaba S. Emulsion drying synthesis of olivine LiFePO4/C composite and its electrochemical properties as lithium intercalation material [J]. Electrochimica Acta, 2004, 49(24): 4213-4222.
[12]Park K S, Kang K T. Synthesis of LiFePO4 with fine particle by co-precipitation method [J]. Materials Research Bulletin, 2004, 39(12): 1803-1810.
[13]Chung S Y, Blocking J T, Chiang Y M. Electronically conductive phospho-olivines as lithium storage electrodes [J]. Nature Mater, 2002, 2: 123-128.
[14]Ouyang C Y, Shi S Q, Wang Z X, et al. The effect of Cr doping on Li ion diffusion in LiFePO4 from first principles investigations and Monte Carlo simulations [J]. J Phys Condens Matter, 2004, 16: 2265-2272.
[15]Croce F, Epifanio A D, Hassoun J, et al. A novel concept for the synthesis of an improved LiFePO4 lithium battery cathode [J]. Electrochem Solid State Lett, 2002, 5(3): A47-A50.
[16]Prosini P P, Zane D, Pasquali M. Improved electrochemical performance of a LiFePO4-based composite cathode [J]. Electrochim Acta, 2001, 46: 3517-3523.
[17]Huang H, Yin S C, Nazar L F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates [J]. Electrochem Solid State Lett, 2001, 4: A170-A172.
[18]HU Guo-rong, ZHANG Xin-long, PENG Zhong-dong, et al. Synthesis and characterization of LiFePO4/C composite as lithium storage electrodes [J]. Trans Nonferrous Met Soc China, 2004, 14(2): 237-240.
[19]Bard A J, Faulkner L R. Electrochem Methods: Fundamental and Applications [M]. New York: Wiley, 1980. 224.
[20]CHEN Zhao-hui, Dahn J R. Reducing carbon in LiFePO4/C composite electrodes to maximized specific energy, volumetric energy, and tap density [J]. J Electrochem Soc, 2002,149: A224-A229.
[21]Huang H, Yin S C, Nazar L F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates [J]. Electrochem Solid State Lett, 2001,4: A170-A172.
Foundation item: Project(04JJ0388) supported by the Natural Science Foundation of Hunan Province, China
Received date: 2004-11-26; Accepted date: 2005-03-03
Correspondence: HU Guo-rong, Professor, PhD; Tel: +86-731-8830474; E-mail: hgrhsj@263.net


