
J. Cent. South Univ. (2018) 25: 2380-2386
DOI: https://doi.org/10.1007/s11771-018-3922-5

Leaching of chalcopyrite: An emphasis on effect of copper and iron ions
YANG Cong-ren(杨聪仁)1, 2, JIAO Fen(焦芬)1, 2, QIN Wen-qing(覃文庆)1, 2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Hunan Province for Clean and Efficient Utilization of Strategic Calcium-containing Mineral Resources, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
Many researchers found that the Fe2+ together with less amount of Cu2+ can accelerate the leaching of chalcopyrite. In this work, the leaching of chalcopyrite with Cu2+ was investigated. The leaching residuals were examined by Raman spectroscopy. Based on the leaching experiments, the chemical equilibrium in solution was calculated using Visual MINTEQ. The results showed that the Fe in chalcopyrite lattice was replaced by Cu2+; therefore, the chalcopyrite transformed into covellite. Furthermore, the formation of chalcocite occurred when Fe2+ and Fe3+ were added to the solution containing Cu2+. The copper extraction increased with a decrease of the initial redox potential (or the ratio of Fe3+/Fe2+).
Key words:
chalcopyrite; covellite; chalcocite; leaching; redox potential;
Cite this article as:
YANG Cong-ren, JIAO Fen, QIN Wen-qing. Leaching of chalcopyrite: An emphasis on effect of copper and iron ions [J]. Journal of Central South University, 2018, 25(10): 2380–2386.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3922-51 Introduction
The rapid consumption of nonferrous metals not only accelerates the depletion of natural resources, but also causes serious environmental problems, therefore, it is necessary to develop efficient recycling technologies [1, 2] and pollution prevention technologies [3–8]. Copper is closely related to people’s lives, and more than 80% of the copper is produced by chalcopyrite [9]. Currently, hydrometallurgy of chalcopyrite, including chemical leaching and bioleaching, has been extensively studied [10–14]. However, the copper extracted from chalcopyrite by hydrometallurgical processes is challenging owing to the passivation of the mineral surface [15–19]. In order to improve the leaching of chalcopyrite, the impact of solution compositions (such as Cu2+, Fe2+, Fe3+) on chalcopyrite leaching has been extensively investigated. In 1974, the anodic dissolution of chalcopyrite was examined in chloride and sulphate solution by JONES [20]. The leaching of chalcopyrite with Fe2(SO4)3 is under mixed control; the leaching of chalcopyrite with CuCl2 or FeCl3 is under anodic control. Since the 2000s, HIROYOSHI et al [21–24] found that coexisting Cu2+ and Fe2+ accelerated chalcopyrite leaching at low redox potential. And a two-step dissolution model for chalcopyrite leaching at low redox potential was proposed: reduction of chalcopyrite to chalcocite by Fe2+ firstly (Eq. (1)), and then oxidation of the formed chalcocite to Cu2+ by Fe3+ (Eq. (2)). Recently, ZHAO et al [25] suggested that leaching of chalcopyrite can be accelerated in an optimum range of redox potential (EH-EL), where the EH depended on the concentration of Cu2+ and Fe2+, the EL depended on the Cu2+ concentration. Similar results were also obtained by YANG et al [26] in the potential range of 350 to 480 mV (vs Ag/AgCl).
 (1)
(1)
 (2)
(2)
The electrochemical results showed that chalcopyrite was reduced to bornite and/or chalcocite in the cathodic, and then formed chalcocite was oxidized to covellite in the anodic [27–29]. Furthermore, bornite, chalcocite, and covellite were detected during the bioleaching of chalcopyrite [26, 30, 31].
In this work, the leaching of chalcopyrite with Cu2+ was conducted at 50 °C. Furthermore, the impact of Fe2+ and Fe3+ on chalcopyrite leaching with Cu2+ was also investigated. The leaching residuals were examined by Raman spectroscopy. Based on the leaching experiments, the chemical equilibrium in solution was calculated using Visual MINTEQ 3.0.
2 Materials and methods
The high grade natural chalcopyrite used in this study was obtained from Daye, Hubei Province, China. The chalcopyrite sample consists of 33.91% Cu, 30.62% Fe, 32.90% S, 0.039% Pb, 0.018% Zn and 6.91 g/t Ag. All chemicals used in this work were analytical grade. Deionized water was used throughout the experiment.
2 g of chalcopyrite powder (100% passing 0.074 mm) and 100 mL of sulfuric acid solutions (pH=1.6) were added into 250 mL flasks, and specific metal ions (Cu2+, Fe2+, and Fe3+) concentrations were also added into solution (Table 1). The leaching experiments were conducted at 50 °C with a speed of 160 r/min. Water lost by evaporation was supplemented periodically by adding deionized water until the mass of the flask equaled its initial mass. The solution pH was adjusted periodically to 1.6 with diluted sulfuric acid. The concentrations of Cu and Fe in solution were analyzed by inductively- coupled plasma-atomic emission spectrometry (ICP-AES) (America Baird Co. PS-6). The solution pH was measured with a pH meter (PHSJ-4A) and the solution potentials were measured with a saturated Ag/AgCl reference electrode and a Pt electrode. After the leaching, the leaching residuals were washed with sulfuric acid solutions of pH 1.6 and dried in a vacuum box at 50 °C. The solid samples were examined by Raman spectroscopy with 633 nm He-Ne laser (Horiba Jobin Yvon Labram HR800). The chemical equilibrium in solution was calculated using Visual MINTEQ 3.0 at 50 °C. The redox couples of Cu+/Cu2+, Fe2+/Fe3+, and HS-/SO42- were chosen.
Table 1 Parameters of leaching experiments

3 Results and discussion
3.1 Effect of Cu2+ ion on chalcopyrite leaching
Figure 1 shows the change of total copper concentration, total iron concentration, and redox potential during the leaching of chalcopyrite with different Cu2+ concentrations. Figure 1(a) indicates that, when the addition of Cu2+ concentration was no more than 0.02 mol/L, the copper concentration in solution increased with time. But, when 0.05 and 0.1 mol/L Cu2+ was added into the solution, the concentrations of copper were less than the initial value during most of the leaching time, meaning the precipitation of the Cu2+ as copper compounds. While Fe concentration increased with the time and reached 0.3 g/L after 14 d of leaching (Figure 1(b)). During the whole leaching process, redox potential was maintained at about 560 mV (Figure 1(c)). The same experimental phenomenon was also observed by ZIES [32]. The results of solution chemistry calculation indicated that the chalcopyrite could transform into covellite via the substitute Fe2+ by Cu2+, as shown in Eq. (3) (Figure 1(d)).
 (3)
(3)
Raman spectra of the original CuFeS2 and leaching residuals were shown in Figure 2. The original CuFeS2 has an intense band at 290 cm-1 and three low intensity bands at 319, 351 and 375 cm-1, which are in agreement with observing by MAJUSTE et al [19]. When chalcopyrite was leached in blank solution, three additional bands were detected at 152, 217 and 471 cm-1, and these bands can be assigned to element sulfur (153, 219 and 472 cm-1). The additional band which was detected 468 cm-1 after chalcopyrite leaching with 0.1 mol/L Cu2+ can be assigned to CuS.
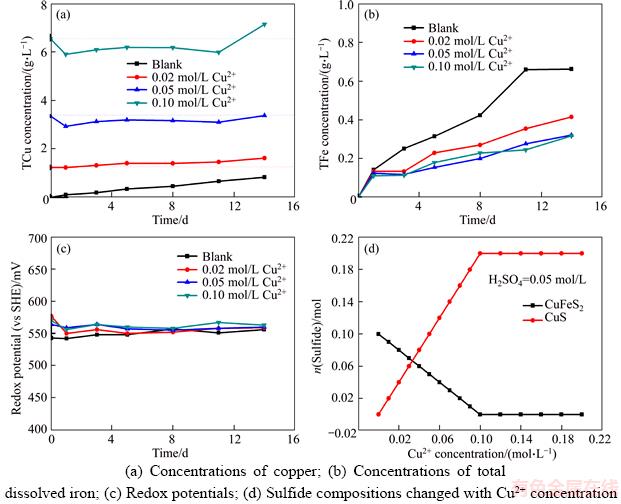
Figure 1 Effect of Cu2+ ion on leaching of chalcopyrite:(Calculation conditions: 0.1 mol of chalcopyrite in 1 L solution)
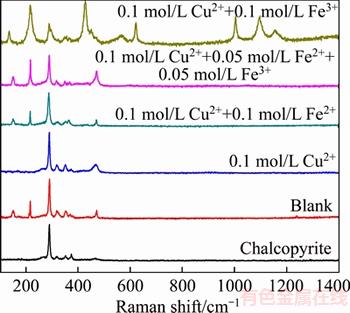
Figure 2 Raman spectra of chalcopyrite and leaching residuals
3.2 Enhancement of chalcopyrite leaching by Fe2+
Figure 3(a) shows that copper extraction increased with the decrease of Fe3+ concentration. In 0.1 mol/L Cu2++0.1 mol/L Fe2+ and 0.1 mol/L Cu2++0.05 mol/L Fe2++0.05 mol/L Fe3+ solutions, the leaching behaviors of Cu and Fe were similar. The concentrations of copper in solution were less than the initial value after 1 d of leaching, and then the copper extraction increased with time. The total iron concentration increased owing to the dissolution of chalcopyrite, while decreased due to the precipitation of Fe3+ as shown in Eq. (4)(Figure 3(b)) [18, 33]. In 0.1 mol/L Cu2++0.1 mol/L Fe2+ leaching system, oxidation of Fe2+ to Fe3+ by dissolved oxygen caused an increase of redox potential from 520 mV to 580 mV in first 3 d. But in 0.1 mol/L Cu2++0.05 mol/L Fe2++0.05 mol/L Fe3+ solutions, the redox potential decreased from 640 mV to 590 mV due to the precipitation of the Fe3+ (Eq. (4)) and/or reduction Fe3+ to Fe2+ (Eq. (5)). In 0.1 mol/L Cu2++0.1 mol/L Fe3+ solutions, the concentrations of copper in solution were almost maintained at its initial value during the whole leaching process. The total iron concentration decreased from 4.8 g/L to 3.5 g/L due to the precipitation of Fe3+ as shown in Eq. (4). Simultaneously, the redox potential decreased from 730 mV to 590 mV due to the precipitation of Fe3+ and/or reduction Fe3+ to Fe2+ [27].

Figure 3 Effect of Cu2+, Fe2+ and Fe3+ ion on leaching of chalcopyrite:
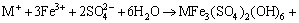
 (4)
(4)
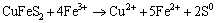 (5)
(5)
Similar to the blank (Figure 2), the element sulfur was also detected by Raman spectroscopy when chalcopyrite was leached with 0.1 mol/L Cu2++0.1 mol/L Fe2+ and 0.1 mol/L Cu2++0.05 mol/L Fe2++0.05 mol/L (Figure 2). Jarosite, which has five intense bands at 222, 433, 624, 1006, 1100 cm-1 and three low intensity bands was at 298, 572, 1151 cm-1, respectively, was detected when chalcopyrite leached in 0.1 mol/L Cu2+ + 0.1 mol/L Fe3+ solution (Figure 2).
Figure 4(a) shows that the redox potential is proportional to the lg(Fe3+/Fe2+), and independent of the Cu2+. Therefore, the redox potential can be used to indicate the change of Fe3+ and Fe2+. The impact of the ratio of Fe3+/Fe2+ on sulfide compositions in the solution was shown in Figure 4(b). Compared to Figure 1(d), the chalcocite was formed when Fe3+ and Fe2+ were added to the solution. Furthermore, chalcopyrite, covellite, and chalcocite coexisted in solution when the concentration of Fe2+ was higher than 8.00× 10–2 mol/L (or concentration of Fe3+ was less than 2.00×10–2 mol/L). When the concentration of Fe2+ decreased from 9.99×10–2 to 8.00×10–2 mol/L, the content of chalcopyrite and chalcocite decreased from 6.73×10–3 to 0 mol and 8.99×10–3 to 3.33×10–3 mol, respectively, but the content of covellite increased from 0.175 to 0.193 mol. And then, the content of covellite decreased from 0.193 to 0.167 mol while the content of chalcocite increased from 3.33×10–3 to 1.67×10–2 mol with a decrease of concentration of Fe2+ from 8.00×10–2 to 1.00×10–4 mol/L. The dissolution of sulfur increased from 2.27×10–3 to 1.67×10–2 mol with a decrease of concentration of Fe2+ from 9.99×10–2 to 1.00×10–4 mol/L (Figure 4(c)). The balance redox potential in solution is shown in Figure 4(d).
The results of leaching experiment showed that the copper extraction decreased with an increase of Fe3+ concentration, and this is in agreement with the previous study [25–27]. HIROYOSHI et al [21–23, 34] suggested that chalcopyrite was firstly reduced to chalcocite when Fe2+ ions and fewer amounts of Cu2+ ions presented in leaching solution, and then the formed chalcocite was oxidized by Fe3+. Figure 4(b) shows that chalcopyrite, covellite, and chalcocite coexisted in solution when the concentration of Fe2+ was higher than 8.00×10–2 mol/L and concentration of Fe3+ was less than 2.00×10–2 mol/L (The redox potential< 630 mV). Figure 4(c) also shows that the dissolution of sulfur increased with an increase of Fe3+ concentration. But during the leaching of chalcopyrite with Fe3+ or high redox potential solution, only less amount of Cu was extracted owing to the formation of passive films consisted of disulfide and polysulfide [18].
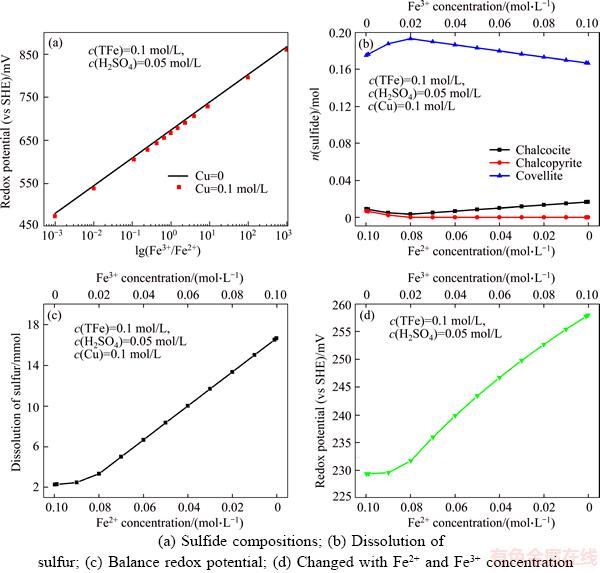
Figure 4 Relationship between the redox potential and ratio of Fe3+/Fe2+:(Calculation conditions: 0.1 mol of chalcopyrite in 1 L solution)
4 Conclusions
The Cu2+ replaced the Fe in chalcopyrite lattice and converted into covellite, thus the decrease of copper concentration was observed. The Fe2+ and Fe3+ played a key role in the formation of chalcocite during the leaching of chalcopyrite with Cu2+. And the Fe2+ ions significantly accelerated the leaching of chalcopyrite. The concentration of copper for the leaching of chalcopyrite with Cu2+ and Fe3+ ions was less than initial value after 14 d; in comparison, a significant amount of copper was leached with Cu2+ and Fe2+ ions.
References
[1] WU Ai-xiang, HU Kai-jian, WANG Hong-jiang, ZHANG Ai-qing, YANG Ying. Effect of ultraviolet mutagenesis on heterotrophic strain mutation and bioleaching of low grade copper ore [J]. Journal of Central South University, 2017, 24: 2245-2252.DOI: 10.1007/s11771-017-3634-2.
[2] AI Chun-ming, WU Ai-xiang, WANG Yi-ming, HOU Chun-lai. Optimization and mechanism of surfactant accelerating leaching test [J]. Journal of Central South University, 2016, 23: 1032-1039. DOI: 10.1007/s11771- 016-0352-0
[3] YANG Jin-qin, YAN Yu-chen, HU Ke-ren, ZHANG Guan-shi, JIANG Dong-yi, LI Qing-zhu, YE Bin, CHAI Li-yuan, WANG Qing-wei, LIU Hui, XIAO Rui-yang. Structural substitution for SO4 group in tooeleite crystal by As(V) and As(III) oxoanions and the environmental implications [J]. Chemosphere, 2018, DOI: 10.1016/ j.chemosphere.2018.09.040.
[4] KONG Xiang-feng, TIAN Tao, XUE Sheng-guo, HARTLEY W, HUANG Long-bin, WU Chuan, LI Chu-xuan. Development of alkaline electrochemical characteristics demonstrates soil formation in bauxite residue undergoing natural rehabilitation [J]. Land Degradation & Development, 2018, 29(1): 58–67. DOI: 10.1002/ldr.2836.
[5] ZHU Feng, LIAO Jia-xin, XUE Sheng-guo, HARTELY W, ZOU Qi, WU Hao. Evaluation of aggregate microstructures following natural regeneration in bauxite residue as characterized by synchrotron-based X-ray micro-computed tomography [J]. Science of the Total Environment, 2016, 573(24): 155–163. DOI: 10.1016/j.scitotenv.2016.08.108.
[6] YAN Xu, CHAI Li-yuan, LI Qing-zhu, YE Li-jun, YANG Ben-tao, WANG Qing-wei. Abiological granular sludge formation benefit for heavy metal wastewater treatment using sulfide precipitation [J]. CLEAN-Soil, Air, Water, 2017, 45(4):1500730.DOI:10.1002/clen.201500730
[7] LIU De-gang, MIN Xiao-bo, KE Yong, CHAI Li-yuan, LIANG Yan-jie, LI Yuan-cheng, YAO Li-wei, WANG Zhong-bing. Co-treatment of flotation waste, neutralization sludge, and arsenic-containing gypsum sludge from copper smelting: Solidification/stabilization of arsenic and heavy metals with minimal cement clinker [J]. Environmental Science and Pollution Research, 2018, 25(8): 7600–7607. DOI: 10.1007/s11356-017-1084-x
[8] MIN Xiao-bo, LI Yang-wen-jun, KE Yong, SHI Mei-qing, CHAI Li-yuan, XUE Ke. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal [J]. Water Science and Technology, 2017, 76(1): 192–200. DOI: 10.2166/wst.2017. 204
[9] de OLIVEIRA C, de LIMA G F, de ABREU H A, DUARTE H A. Reconstruction of the chalcopyrite surfaces—A DFT study [J]. Journal of Physical Chemistry C, 2012, 116(10): 6357–6366. DOI: 10.1021/jp300713z.
[10] TURAN M D, ARSLANO LU H, ALTUNDO
LU H, ALTUNDO AN H S. Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system [J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 50 (Supplement C): 49–55. DOI: 10.1016/ j.jtice.2014.12.009.
AN H S. Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system [J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 50 (Supplement C): 49–55. DOI: 10.1016/ j.jtice.2014.12.009.
[11] PETERSEN J, DIXON D G. Thermophilic heap leaching of a chalcopyrite concentrate [J]. Minerals Engineering, 2002, 15(11): 777–785. DOI: 10.1016/S0892-6875(02)00092-4.
[12] TURAN M D. Direct selective leaching of chalcopyrite concentrate [J]. Canadian Metallurgical Quarterly, 2014, 53(4): 444–449. DOI: 10.1179/1879139514Y.0000000141.
[13] GU Guo-hua, XIONG Xian-xue, HU Ke-ting, LI Shuang-ke, WANG Chong-qing. Stepwise dissolution of chalcopyrite bioleaching by thermophile A. manzaensis and mesophile L. ferriphilum [J]. Journal of Central South University, 2015, 22(10): 3751–3759. DOI: 10.1007/s11771-015-2919-6.
[14] GU Guo-hua, HU Ke-ting, LI Shuang-ke. Bioleaching and electrochemical properties of chalcopyrite by pure and mixed culture of Leptospirillum ferriphilum and Acidthiobacillus thiooxidans [J]. Journal of Central South University, 2013, 20(1): 178–183. DOI: 10.1007/s11771-013-1474-2.
[15] KLAUBER C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution [J]. International Journal of Mineral Processing, 2008, 86(1–4): 1–17. DOI: 10.1016/j.minpro. 2007.09.003.
[16] GHAHREMANINEZHAD A, DIXON D G, ASSELIN E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution [J]. Electrochimica Acta, 2013, 87: 97–112. DOI: 10.1016/j.electacta.2012.07.119.
[17] ACRES R G, HARMER S L, BEATTIE D A. Synchrotron XPS studies of solution exposed chalcopyrite, bornite, and heterogeneous chalcopyrite with bornite [J]. International Journal of Mineral Processing, 2010, 94 (1, 2): 43–51. DOI: 10.1016/j.minpro.2009.11.006.
[18] WU Shi-fa, YANG Cong-ren, QIN Wen-qing, JIAO Fen, WANG Jun, ZHANG Yan-sheng. Sulfur composition on surface of chalcopyrite during its bioleaching at 50 °C [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4110–4118. DOI: 10.1016/S1003-6326(15)64062-6.
[19] MAJUSTE D, CIMINELLI V S T, OSSEO-ASARE K, DANTAS M S S, MAGALHAES-PANIAGO R. Electrochemical dissolution of chalcopyrite: Detection of bornite by synchrotron small angle X-ray diffraction and its correlation with the hindered dissolution process [J]. Hydrometallurgy, 2012, 111: 114–123. DOI: 10.1016/ j.hydromet.2011.11.003.
[20] JONES D L. The leaching of chalcopyrite [D]. Vancouver: University of British Columbia, 1974. DOI: 10.14288/ 1.0078729.
[21] HIROYOSHI N, MIKI H, HIRAJIMA T, TSUNEKAWA M. A model for ferrous-promoted chalcopyrite leaching [J]. Hydrometallurgy, 2000, 57(1): 31–38. DOI: 10.1016/s0304- 386x(00)00089-x.
[22] HIROYOSHI N, MIKI H, HIRAJIMA T, TSUNEKAWA M. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions [J]. Hydrometallurgy, 2001, 60(3): 185–197. DOI: 10.1016/s0304-386x(00)00155-9.
[23] HIROYOSHI N, KUROIWA S, MIKI H, TSUNEKAWA M, HIRAJIMA T. Synergistic effect of cupric and ferrous ions on active-passive behavior in anodic dissolution of chalcopyrite in sulfuric acid solutions [J]. Hydrometallurgy, 2004, 74 (1, 2): 103–116. DOI: 10.1016/j.hydromet. 2004.01.003.
[24] HIROYOSHI N, KITAGAWA H, TSUNEKAWA M. Effect of solution composition on the optimum redox potential for chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2008, 91(1–4): 144–149. DOI: 10.1016/ j.hydromet.2007.12.005.
[25] ZHAO Hong-bo, WANG Jun, YANG Cong-ren, HU Ming-hao, GAN Xiao-wen, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Effect of redox potential on bioleaching of chalcopyrite by moderately thermophilic bacteria: An emphasis on solution compositions [J]. Hydrometallurgy, 2015, 151: 141–150. DOI: 10.1016/j.hydromet.2014.11.009.
[26] YANG Yi, LIU Wei-hua, CHEN Miao. XANES and XRD study of the effect of ferrous and ferric ions on chalcopyrite bioleaching at 30 °C and 48 °C [J]. Minerals Engineering, 2015, 70: 99–108. DOI: 10.1016/j.mineng.2014.08.021.
[27] QIN Wen-qing, YANG Cong-ren, LAI Shao-shi, WANG Jun, LIU Kai, ZHANG Bo. Bioleaching of chalcopyrite by moderately thermophilic microorganisms [J]. Bioresource Technology, 2013, 129: 200–208. DOI: 10.1016/j.biortech. 2012.11.050.
[28] GU Guo-hua, HU Ke-ting, ZHANG Xun, XIONG Xian-xue, YANG Hui-sha. The stepwise dissolution of chalcopyrite bioleached by Leptospirillum ferriphilum [J]. Electrochimica Acta, 2013, 103: 50–57. DOI: 10.1016/j.electacta. 2013.04.051.
[29] LIANG Chang-li, XIA Jin-lan, YANG Yi, NIE Zhen-yuan, ZHAO Xiao-juan, ZHENG Lei, MA Chen-yan, ZHAO Yi-dong. Characterization of the thermo-reduction process of chalcopyrite at 65 °C by cyclic voltammetry and XANES spectroscopy [J]. Hydrometallurgy, 2011, 107(1, 2): 13–21. DOI: 10.1016/j.hydromet.2011.01.011.
[30] LIU Hong-chang, NIE Zhen-yuan, XIA Jin-lan, ZHU Hong-rui, YANG Yun, ZHAO Chang-hui, ZHENG Lei, ZHAO Yi-dong. Investigation of copper, iron and sulfur speciation during bioleaching of chalcopyrite by moderate thermophile Sulfobacillus thermosulfidooxidans [J]. International Journal of Mineral Processing, 2015, 137: 1–8. DOI: 10.1016/j.minpro.2015.02.008.
[31] LIU Hong-chang, XIA Jin-lan, NIE Zhen-yuan. Relatedness of Cu and Fe speciation to chalcopyrite bioleaching by Acidithiobacillus ferrooxidans [J]. Hydrometallurgy, 2015, 156: 40–46. DOI: 10.1016/j.hydromet.2015.05.013.
[32] ZIES E G. Some reactions involved in secondary copper sulphide enrichment [J]. Economic Geology, 1916, 11(5): 407–503. DOI: 10.2113/gsecongeo.11.5.407.
[33] YANG Cong-ren, QIN Wen-qing, LAI Shao-shi, WANG Jun, ZHANG Yan-sheng, JIAO Fen, REN Liu-yi, ZHUANG Tian, CHANG Zi-yong. Bioleaching of a low grade nickel– copper–cobalt sulfide ore [J]. Hydrometallurgy, 2011, 106(1, 2): 32–37. DOI: 10.1016/j.hydromet.2010.11.013.
[34] HIROYOSHI N, HIROTA M, HIRAJIMA T, TSUNEKAWA M. A case of ferrous sulfate addition enhancing chalcopyrite leaching [J]. Hydrometallurgy, 1997, 47(1): 37–45. DOI: 10.1016/s0304-386x(97)00032-7.
(Edited by HE Yun-bin)
中文导读
黄铜矿浸出:强调铜离子和铁离子的作用
摘要:许多研究发现,浸出液中添加 Fe2+ 和少量的 Cu2+ 离子可以促进黄铜矿的浸出。研究了 Cu2+ 离子的添加对黄铜矿浸出的影响,并通过Raman 光谱表征了黄铜矿浸出过程中形成的中间产物。同时,利用 Visual MINTEQ 软件模拟计算了浸出体系的化学平衡。模拟计算结果表明:黄铜矿晶格中的Fe原子由于被 Cu2+ 取代而转变为铜蓝;此外,当 Fe2+ 和 Fe3+ 离子添加到含有 Cu2+ 离子的黄铜矿浸出体系中会导致辉铜矿的形成。浸出试验结果表明:铜的浸出率随着初始氧化还原电位(或Fe3+/Fe2+ 的比例)的降低而增加。
关键词:黄铜矿;铜蓝;辉铜矿;浸出;氧化还原电位
Foundation item: Project(2016RS2016) supported by the Hunan Provincial Science and Technology Leader (Innovation Team of Interface Chemistry of Efficient and Clean Utilization of Complex Mineral Resources), China; Project supported by the Co-Innovation Centre for Clean and Efficient Utilization of Strategic Metal Mineral Resources, China; Project(2015CX005) supported by the Innovation Driven Plan of Central South University, China
Received date: 2017-09-26; Accepted date: 2017-12-18
Corresponding author: QIN Wen-qing, PhD, Professor; Tel: +86–731–88830884; E-mail: qinwenqing369@126.com; ORCID: 0000- 0001-5570-9680
Abstract: Many researchers found that the Fe2+ together with less amount of Cu2+ can accelerate the leaching of chalcopyrite. In this work, the leaching of chalcopyrite with Cu2+ was investigated. The leaching residuals were examined by Raman spectroscopy. Based on the leaching experiments, the chemical equilibrium in solution was calculated using Visual MINTEQ. The results showed that the Fe in chalcopyrite lattice was replaced by Cu2+; therefore, the chalcopyrite transformed into covellite. Furthermore, the formation of chalcocite occurred when Fe2+ and Fe3+ were added to the solution containing Cu2+. The copper extraction increased with a decrease of the initial redox potential (or the ratio of Fe3+/Fe2+).


