Effects of surface modification on electrochemical performance of MnO2
LIU Li-qing(刘立清)1, WANG Jian-ming(王建明)1, FAN Yu-kai(范玉凯)1,
WANG Guo-guang(王国光)1, ZHANG Jian-qing(张鉴清)1, 2
(1. Department of Chemistry, Zhejiang University, Hangzhou 310027, China;
2. State Key Laboratory for Corrosion and Protection, Institute of Metal Research,Chinese Academy of Sciences, Shenyang 110015, China)
Abstract:
The MnO2 samples coated with Ca(OH)2 were prepared by a liquid-phase surface treatment method. The physical properties of the samples were examined by SEM, EDAX and chemical analysis, and their electrochemical performances were investigated by means of galvanostatic charge-discharge, cyclic voltammetry and electrochemical impedance spectroscopy (EIS). The SEM results show that the samples coated with Ca(OH)2 display a porous surface structure. The electrochemical experiments indicate that the surface modification decreases the polarization of MnO2 electrodes and improves their discharge potentials and discharge capacities.
Key words:
manganese dioxide; surface modification; cyclic voltammetry; electrochemical impedance spectroscopy CLC number: TM911;
Document code: A
1 INTRODUCTION
MnO2 is widely used as electrode materials in primary batteries due to its low cost, environmentally friendly nature and good electrochemical performance[1-4]. As the main cathode material of alkaline batteries, the properties of MnO2 are crucial to the electrochemical performance of the batteries. MnO2 has attracted numerous attentions of researchers since its early discovery as a cathode material in Leclanche type dry cell[4, 5]. In order to overcome the drawbacks of MnO2, such as the bad reversibility and easy overcharge, the researchers have extensively investigated the effects of various additives such as Fe, Ti, Bi, Ba, Pb, Ni, Co, and V[6-12] on the electrochemical performance of MnO2. It has been found that Bi, Ba and Pb can improve the reversibility of MnO2, and the additions of Co and Ni are advantageous to avoiding the overcharge of MnO2 electrodes. Although the electrochemical performance of MnO2 has been greatly improved in the past several decades, its rate capability and electrochemical reversibility need to be further improved to meet the actual increasing requirements.
The surface modification is a novel method to improve the properties of materials[13, 14]. Wus results[13] showed that the electrochemical performance of Ni(OH)2 powder was obviously improved by the surface treatment. However, few studies have been carried out in the surface modification of MnO2.
In this paper, the effects of the surface modification using Ca(OH)2 on the physical properties and electrochemical performance of MnO2 were investigated.
2 EXPERIMENTAL
2.1 Preparation of samples
With continual stirring, the Ca(NO3)2 solution (0.25mol/L) and KOH solution (0.2mol/L) were slowly added to the well-sealed reaction vessel with 20mL ammonia solution (6.47mol/L), 0.5mol commercial MnO2 and appropriate amount of Tween-20. The reaction temperature was controlled at (40±1)℃, and the pH value of the reaction solution was held at (12.50±0.1). After the reaction had been undertaken for certain time, the reaction product was aged in the mother solution for 2h at (40±1)℃.The product was filtered off, rinsed several times using deionized water, and dried at 60℃ in air.
When the addition amount of Ca2+ was 0.1, 0.05, 0.033 and 0.025mol, the corresponding product obtained was labeled with A, B, C, and D, respectively. Commercial MnO2 was designated as E.
2.2 Physical characterization of samples
The MnO2 contents in samples were determined by the ferrous sulphate reduction method[2]. The morphologies and surface compositions of the samples were examined by scanning electron microscopy (SEM) and EDAX with an FEI SIRION system and Link-Isis analyzer.
2.3 Preparation of electrodes and their electrochemical measurements
The pasted manganese electrodes were prepared as follows: 160mg of MnO2 and 80mg of graphite powder were thoroughly mixed with a certain amount of 5%(mass fraction) PTFE solution. The paste obtained was incorporated into a nickel foam (2cm×2cm×1.1mm) with a spatula. The pasted electrodes were dried at 60℃ and then roll-pressed to a thickness of 0.5mm. Thereafter, the electrodes were soaked in 9mol/L KOH for 8h before being coupled with Ni electrodes on either side as the counter electrodes and an Hg/HgO electrode as reference.
Galvanostatic charge-discharge studies were conducted with BT-2000 Arbin battery testing instrument. The primary discharge was undertaken from open-circuit potential to -1.0V(vs Hg/HgO) at constant currents of 62.5mA/g and 250mA/g, respectively. In the charge-discharge experiments the pasted manganese electrodes were galvanostatically discharged to -0.2V at rate of 62.5mA/g, and then charged to 0.5V at rate of 31.25mA/g. Electrochemical impedance spectroscopy (EIS) measurement was performed with a Potentiosta/Galvanostat Model 273 in conjunction with a model 5210 lock-in amplifier. The frequency range was from 120kHz to 5mHz, and the excitation amplitude was 10mV.
Cyclic voltammetry experiment was performed with a Potentiosta/Galvanostat Model 273 potentiosta within a potential range from -1.0V to 0.6V(vs Hg/HgO) at a rate of 0.5mV/s. The working electrode was cavity microelectrode described in the literature[15]. The counter electrode and the reference electrode were just the same as the above experiments. All the electrochemical measurements were performed at room temperature.
3 RESULTS AND DISCUSSION
3.1 Physical properties of samples
The SEM images of the samples are illustrated in Fig.1. The morphology of MnO2 sample is obviously altered by the surface treatment. Compared with the untreated sample (E), the treated sample (B) shows more regular shape and porous surface, which is advantageous to improving the electrochemical activity of MnO2. The results of EDAX show that the surface of Sample B consists of manganese, oxygen and calcium. This indicates that Ca(OH)2 is coated on the surface of MnO2 during the treatment process of MnO2 in the Ca-containing alkaline solution.
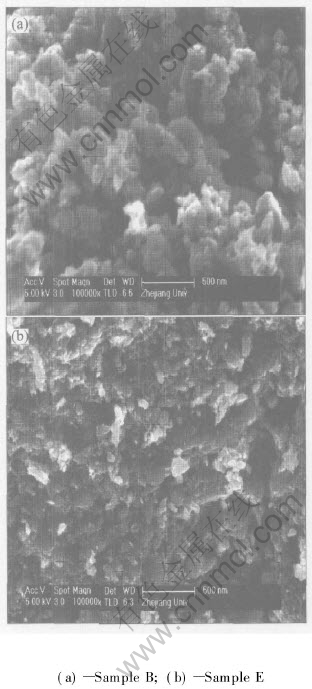
Fig.1 SEM images of samples
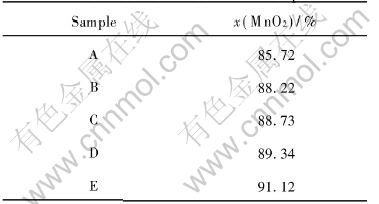
The MnO2 contents in various samples are displayed in Table 1. It can be seen that as the addition amount of Ca2+ in the treatment solution increases, the MnO2 content in the samples decreases. This implies that the amount of Ca(OH)2 coated on the surface of MnO2 increases with the increasing content of Ca2+ in the treatment solution.
Table 1 MnO2 contents in samples
3.2 Electrochemical performance of samples
The primary discharge curves of the samples at different rates are displayed in Fig.2. It can be seen that the samples coated with Ca(OH)2 show larger discharge capacity and higher discharge potential at both low and high rates than the untreated sample, and relatively higher calcium content is advantageous to improving the electrochemical performance of the MnO2 samples. The beneficial effects of the surface modification using Ca(OH)2 on the electrochemical performance of the samples can be attributed to the formation of the porous surface structure, which can enhance the diffusion of electrolyte within the porous electrodes.
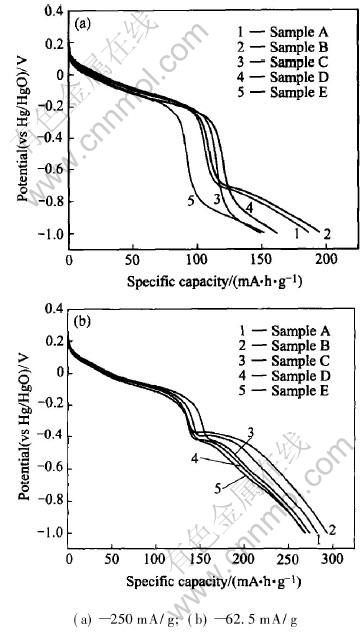
Fig.2 Discharge curves of samples at different rates
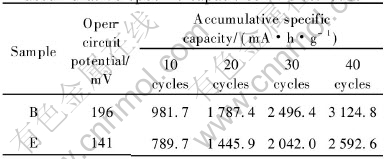
The cyclic behaviors of the manganese electrodes with the treated (B) and untreated (E) surface are illustrated in Fig.3. In accordance with Ref.[16], the discharge capacities of both Sample B and Sample E have a relatively large deterioration rate during the whole cycle, but Sample B has larger discharge capacity than Sample E at all cycles. The data in Table 2 indicate that the surface modification using Ca(OH)2 markedly increases the open-circuit potential and accumulative discharge capacity of MnO2 sample.
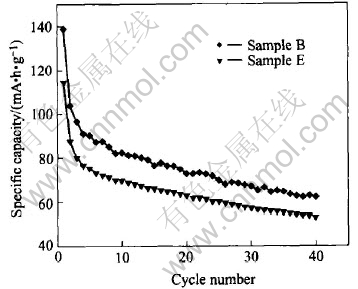
Fig.3 Cyclic performance of samples
Table 2 Open-circuit potentials and accumulative specific capacities of electrodes
Cyclic voltammetry measurement results are displayed in Fig.4. During the cathodic sweep, the first cathodic peak (-0.2V) represents the one electron reduction of Mn(Ⅳ), which corresponds to the homogeneous reaction in the solid phase of Mn(Ⅳ)→Mn(Ⅲ). The second strong cathodic peak (-0.44V) represents the reduction of the Mn(Ⅲ), which corresponds to the heterogeneous phase reaction[8]. The curves of samples B and E are similar. The similar position and shape of peaks imply that the surface modification does not change the electrochemical reaction mechanism of MnO2. It can be found from Fig.4 that Sample B exhibits higher cathodic peak current than Sample E, indicating that the sample coated by Ca(OH)2 has higher electrochemical activity.
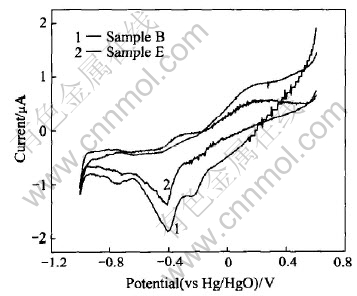
Fig.4 Cyclic voltammograms of Sample B and Sample E
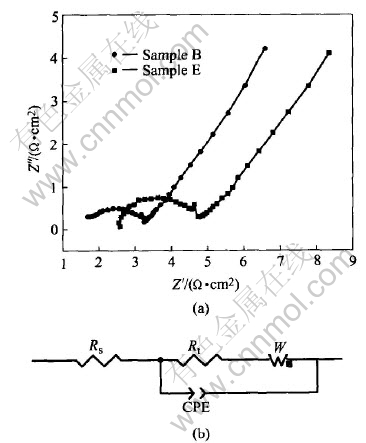
Fig.5 Nyquist plots for Samples B and E at open-circuit potentials(a) and corresponding equivalent circuit(b)
In order to investigate the effects of Ca(OH)2 treatment on the kinetic process of MnO2 electrodes, electrochemical impedance spectroscopy (EIS) of the electrodes was measured at open-circuit potentials. The Nyquist plots of samples B and E are displayed in Fig.5(a). The plots show a semicircle in high-frequency region due to the charge transfer resistance parallel with the double layer capacitance; and a straight line in low-frequency region. The radius size of the semicircle reflects the magnitude of the charge-transfer resistance; and the straight line generally originates from solid phase proton diffusion, which reflects the capability of proton diffusion. This system can be depicted by the equivalent circuit shown in Fig.5(b), where Rs is the total ohmic resistance of the electrode system, CPE the constant phase element related to the double layer capacity, Rt charge-transfer resistance, W the Warburg impedance (Zw) of the solid phase diffusion[17, 18]. Table 3 lists the fitting parameters (Rs, Rt, Ws-R and Ws-T) of EIS, where Ws-T= L2/D, L is the effective diffusion thickness and D the effective diffusion coefficient. It can be seen from Table 3 that Rs, Rt, Ws-R and Ws-T values of the sample coated with Ca(OH)2 decrease. This indicates that the ohmic, electrochemical and diffusion polarizations of MnO2 electrodes are all decreased by the surface modification with Ca(OH)2. Therefore, the MnO2 samples with Ca(OH)2 coating have higher electrochemical activity, larger discharge capacity and relatively better electrochemical reversibility.
Table 3 Simulation results for EIS of electrodes

4 CONCLUSIONS
1) The MnO2 samples coated by Ca(OH)2 are obtained by a liquid-phase surface modification method. The results of SEM show that the samples coated by Ca(OH)2 have more regular shape and porous surface structure.
2) The electrodes of MnO2 coated by Ca(OH)2 display lower ohmic, electrochemical and diffusion polarizations. This results in higher electrochemical activity, larger discharge capacity and relatively better electrochemical reversibility of the MnO2 samples coated with Ca(OH)2.
REFERENCES
[1]Kannan A M, Bhavaraju S, Prado F, et al. Characterization of the Bismuth-modified manganese dioxide cathodes in rechargeable alkaline cells[J]. Journal of Electrochemical Society, 2002, 149(4): A483-A492.
[2]Kordesch K V. Batteries(Vol.1), Manganese Dioxide[M]. New York: Marcel Dekker, 1974. 31-34.
[3]Hu C C, Wang C C. Nanostructure and capacitive characteristics of hydrous manganese dioxide prepared by electrochemical deposition[J]. Journal of Electrochemical Society, 2003, 150(8): A1079-A1084.
[4]Chin S F, Pang S C, Anderson M A. Material and electrochemical characterization of tetrapropylammonium manganese oxide thin films as novel electrode material for electrochemical capacitors[J]. Journal of Electrochemical Society, 2002, 149(4): A379-A384.
[5]Reddy R N, Reddy R G. Synthesis and electrochemical characterization of amorphous MnO2 electrochemical capacitor electrode material[J]. Journal of Power Sources, 2004, 132: 315-320.
[6]Fernandes J B, Desai B D, Kamatdalal V N. Effect of chemically dopants/impurities on the discharge behaviour of chemical manganese dioxide in alkaline medium and the applicability of Atlung-Jacobsen model to the (MnO2)1-r(MnOOH)r system[J]. Journal of Applied Electrochemistry, 1985, 15: 351-363.
[7]Klob M, Rahner D, Plieth W. The effect of alkaline earth titanates on the rechargeability of manganese dioxide in alkaline electrolyte[J]. Journal of Power Sources, 1997, 69: 137-143.
[8]Nartey V K, Binder L, Huber A. Production and characterization of titanium doped electrolytic manganese dioxide for use in rechargeable alkaline zine/manganese dioxide batteries[J]. Journal of Power Sources, 2000, 87: 205-211.
[9]TONG Qing-song, LIAN Jing-ming. The physicochemical properties of MnO2 prepared from MnCl2 solution by an electrochemical codeposition method[J]. Journal of Electrochemical Society, 1997, 144(2): 4111-4118.
[10]Im D, Manthiram A, Coffey B. Manganese(Ⅲ) chemistry in KOH solution in the presence of Bi- or Ba-containing compounds and its implications on the rechargeability of γ-MnO2 in alkaline cells[J]. Journal of Electrochemical Society, 2003, 150(12): A1651-1659.
[11]Bode M, cachet C, Bach S, et al. Rechargeability of MnO2 in KOH media produced by decomposition of dissolve KMnO4 and Bi(NO)3 mixtures[J]. Journal of Electrochemical Society, 1997, 144(3): 792-801.
[12]WANG Jia-xiong, ZHU Ze-shan, ZHANG Qi-xin. The discharge capacity and some physical and chemical properties of electrolytic manganese dioxide doped with vanadium[J]. Journal of Fujian Normal University(Natural Science), 1987, 3(1): 32-38.
[13]Wu M S, Huang C M, Wang Y Y. Effect of surface modification of hydroxide powder on the electrode performance of nickel/metal hydride batteries[J]. Electrochimica Acta, 1999, 44: 4007-4016.
[14]Jayashree R S, Kamath P V. Modified nickel hydroxide electrodes effect of colat metal on the different polymorphic modifications[J]. Journal of Electrochemical Society, 2002, 149(6): A761-A764.
[15]CHA Quan-xing, LI Chang-ming. Power microelectrode[J]. Journal of Electroanalytical Chemistry, 1994, 368: 47-54.
[16]Ghaemi M, Biglari Z, Binder L. Effect of bath temperature on electrochemical properties of the anodically deposited manganese dioxide[J]. Journal of Power Sources, 2001, 102: 29-34.
[17]Qu D Y. Application of a. c. impedance technique to the study of the proton diffusion process in the porous MnO2 electrode[J]. Electrochimica Acta, 2003, 48: 1675-1684.
[18]CHEN Hui, WANG Jian-ming, PAN Tao, et al. Structure and electrochemical performance of Al and Zn co-substituted α-Ni(OH)2[J]. Trans Nonferrous Met Soc China, 2003, 13 (1): 85-90.
Foundation item: Project (59902004) supported by the National Natural Science Foundation of China
Received date: 2004-11-05; Accepted date:2005-03-21
Correspondence: WANG Jian-ming, Professor, PhD; Tel: +86-571-87951513; Fax: +86-571-87953309;
E-mail: wjm@cmsce.zju.edu.cn


