Article ID: 1003-6326(2005)05-1156-05
Preparation of titanium dioxide/silver sulfate powder and its antibacterial activity
PENG Bing(彭 兵), YUAN Chun(苑 春), CHAI Li-yuan(柴立元),
WEI Shun-wen(韦顺文), YU Yan-fen(于延芬), SU Wei-feng(苏维丰)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
Antibacterial powders of titanium dioxide/silver sulfate were produced by heat-treatment of the metatitanic acid, as precursor, into which the silver nitrate was added. The influences of heating temperature on the structure and composition of the product were investigated through XRD and SEM. The results show that the powder is spherical in the phase of TiO2-Ag2SO4. The granularity of the particles increases from 10.7nm to 28.7nm with the temperature of heat-treatment increasing from 300℃ to 800℃. The antibacterial activity of the powder was judged in the way of the minimum inhibitory contents (MiCs). When the content of silver sulfate is less than 2%, the photocatalysis of titanium dioxide and silver ions cooperate to kill bacteria. And the MiCs decrease and keep around 1.0×10-4-1.5×10-4 constantly with the increase of silver content. Furthermore, the MiCs decrease with the increase of temperature of heat-treatment when the temperature is lower than 500℃. But when the temperature is beyond 600℃ the MiCs increase quickly, which shows the inferior antibacterial performance.
Key words:
titanium dioxide/silver sulfate composite powder; preparation; antibacterial activity; MiCs CLC number: TQ134.1;
Document code: A
1 INTRODUCTION
With the progress of human living condition, more and more attentions are paid to the long asepsis of environment. Research and application of the inorganic antibacterial reagents have been widely conducted because of their advantages in safety, permanence and broad-spectrum[1]. Among these reagents, antibacterial reagent of silver and light catalyst of titanium dioxide are used widely, and main researches on silver antibacterial products are aimed at adding silver ions into zeolite[2] and phosphate[3] carrier. The silver ions combine with thiol groups(—SH), leading to solidification of proteins so that the cytoplasm is shrunk and detached from the cell wall[4]. When the cell loses the activity, silver ions get free from the process and continue to sterilize, which makes the effects of sterilization get longer[5]. But silver ions can not release stably, which restricts their applications in materials. Titanium dioxide has a high photochemical characteristic. The free electron and cavity pairs are generated when TiO2 particles are under the irradiation of ultraviolet. The cavities oxidize water or hydroxide ions to form free hydroxide, which can effectively decompose and mineralize the organic pollutants[6-9]. When bacteria are attached to the surface of titanium dioxide, the cell wall and membrane will be destroyed by the hydrogen peroxide (O-2) and hydroxyl radical(OH), resulting in leakage of the cell contents[10, 11] .The poisonous components are gradated in the process of death of bacteria, for example endotoxin[12, 13]. But it can only react with the light of wavelength under 385nm (UV wavelength)[14]. In order to overcome this disadvantage, silver ions are added to enhance the antibacterial performance. The titanium dioxide/silver sulfate composite antibacterial powder is prepared by adding silver nitrate to TiO2 in this study. And the antibacterial activity of the powder is studied.
2 EXPERIMENTAL
2.1 Experimental materials
The experimental materials used included industrial metatitanic acid, chemically pure sulfuric acid, analytically pure sodium hydroxide and analytically pure silver nitrate.
2.2 Method for antibacterial powder preparation
A certain amount of industrial metatitanic acid was dissolved into hot sulfuric acid (12mol/L) in a molar ratio of 1∶1.2 under continuous agitation. The slurry was filtered and the impurities in the solution were removed. The final concentration of H+ in titanium sulfate solution was adjusted to around 3mol/L. The output rate of metatitanic acid exceeded 90% after reaction for 1h at 125℃. The purified metatitanic acid precursor was obtained by filtrating and washing with distilled water. The stoichiometrical silver nitrate solution(0.25mol/L) was dripped into the precursor under stirring. After 1h, the produced solid powders were filtrated, washed with deionized water, dried under 80℃ and then transferred to a Muffle furnace for heat-treatment. Titanium dioxide/silver sulfate antibacterial powders were then obtained.
2.3 Measurements
The crystal phases were determined by X-ray diffraction using a Rigaku D/max2550VB+ diffractometer with CuKα radiation (40kV, 300mA, 10°≤2θ≤85°). The powder morphology was studied by using scanning electron microscopy (SEM) (KYKY-2800).
2.4 Antibacterial property test
Firstly, the antibacterial powder was enclosed into a test tube filled with 5mL liquid culture medium. After mixing, 0.5mL liquid is exactly measured into another test tube filled with 4.5mL liquid culture medium with a transplanting tube, which makes the concentration of powder dilute by 10 multiple. Then 0.5mL inocula liquids of each tube were cultivated for 24h at 37℃. The MiCs are defined as the lowest concentration of TiO2-Ag2SO4 powder at which the clearness of the nutrient liquid could be kept. The concentration of E. coli used in the experiment was surveyed in advance by counting under a plate agar. The final concentration of the bacteria cell was adjusted to 1×106 cells/mL[15].
3 RESULTS AND DISCUSSION
3.1 Main phases of Ag2SO4-TiO2 powder
3.1.1 Main phases at different heating temperatures
The samples are put in the furnace to calcine when pre-set temperature is reached, in which the heating speed of the samples is fast. Fig.1 shows the XRD patterns of titanium dioxide/silver sulfate obtained at 300-800℃ with the silver content of 1%.
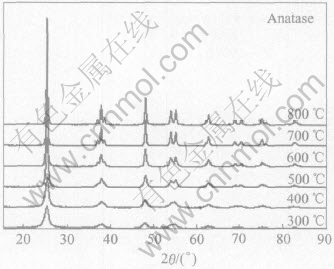
Fig.1 XRD patterns of products obtained at different heating temperatures
It can be seen from Fig.1 that all the samples are in the form of anatase at different heat-treating temperatures. Diffraction peaks of samples are more intense and narrow at higher heating temperature. This means that the average size of grains increases. The anatase is easy to form because of the presence of SO2-4[16]. The perfect anatase crystal still remains even at 800℃ and no evidence indicates that it transforms to rutile. Because the specific surface area of anatase is larger than that of rutile, the recombination of cavity and electron is slow, the capability of adsorption oxygen is high, and the efficiency of photocatalysis is large[18]. So, the acquired titanium dioxide /silver sulfate composite powder produced in this method is stable anatase. This is very important for the powders present in the form of anatase in order to retain the photocatalytic activity.
The average particle size is estimated by analyzing the broadening of the (101) reflection and calculated according to Scherrer equation d=0.9λ/(β cosθ) (where λ is the wavelength of X-ray source (CuKα=1.54056) and β is the full-width at half-maximum (FWHM) of the X-ray diffraction peaks at the diffraction angle θ). Linear regression is used to analyze the obtained values. Fig.2 shows the average diameter of grains at various heating temperatures. The crystal size increases from 10.7nm at 300℃ to 28.7nm at 800℃. This is resulted from the facts that the small grains disappear and bigger grains continuously develop. Considering less grains and complete crystal transform, the temperature is fixed at 500℃.

Fig.2 Profile of particle size under different heating temperatures
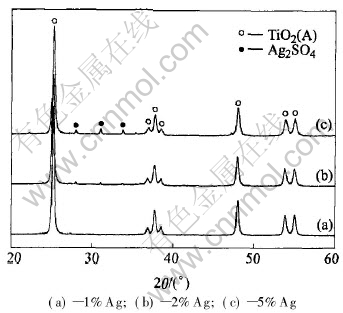
Fig.3 XRD patterns of titanium dioxide with different silver contents after thermal treatment
3.1.2 Phases with different silver contents in titanium dioxide
Fig.3 shows the XRD patterns of titanium dioxide with different silver contents after heat-treatment at 500℃. The patterns of Fig.3(a), (b), (c) correspond to the samples with 1%, 2% and 5% silver contents(mass fraction), respectively. After heat-treatment at 500℃, all of TiO2 present in the powder with different silver contents are in the form of anatase. The characteristic peaks of Ag2SO4 are not observed and only peaks of TiO2 appear when the quantity of Ag2SO4 is small. This results from the fact that Ag2SO4 is evenly dispersed into the pores of TiO2, which leads to the loss of continued phase. But with the increase of Ag2SO4 amount, the crystal state of superfluous Ag2SO4 appears on the surface of TiO2 because of the reuniting of Ag2SO4 so that the patterns of Ag2SO4 get more evident. To prevent much Ag2SO4 from covering on the surface of titanium dioxide and obstructing the irradiation of light, 2% silver content is condign.
3.2 Morphology of TiO2/silver sulfate powders
The SEM morphology of silver sulfate and titanium dioxide composite is shown in Fig.4. It can be seen that the shape of silver sulfate and titanium dioxide composite powder is spherical, and the distribution of particle size is very narrow.
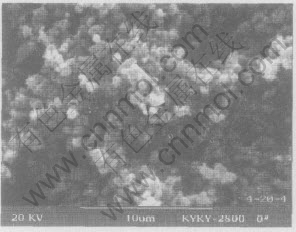
Fig.4 SEM morphology of titanium dioxide doping silver obtained at 500℃
4 ANTIBACTERIAL PROPERTY
4.1 Effect of silver contents on MiCs
The performance of titanium dioxide/silver sulfate is contrasted to that of silicon dioxide/silver sulfate which has no photocatalysis effect. The optimal silver content of titanium dioxide/silver sulfate powder is confirmed by measuring the MiCs. The powder obtained can make use of the photocatalysis of titanium dioxide as well as the antibacterial effect of Ag+. The plot is given in Fig.5.
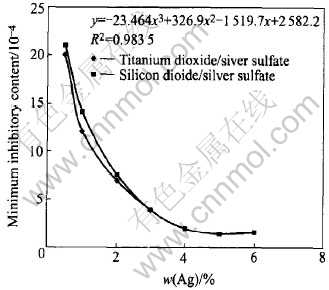
Fig.5 Effect of silver content on MiCs
We can see from Fig.5 that the antibacterial performance of titanium dioxide/silver sulfate is always superior to that of silicon dioxide/silver sulfate when silver contents are less than 2%. It is due to the fact that photocatalysis of titanium dioxide and silver ions cooperate to kill bacilli at lower silver contents. But because the photocatalysis effect is faintish, the MiCs of titanium dioxide/silver sulfate are appreciably lower. When doping silver content is higher, on one hand, the silver ions become the center of recombination of cavity and electron of photocatalysis, and redundant silver sulfate deposits on TiO2 surface, which prevents the light from shining, and both reduce the capacity of photocatalysis of titanium dioxide. On the other hand, the antibacterial performances are operated by Ag+ with the increase of silver contents, which indicates the MiCs of titanium dioxide/silver sulfate and silicon dioxide/silver sulfate are basically consistent. The MiCs finally keep a constant value of 1.0×10-4-1.5×10-4 when the silver content exceeds 5%. This is because that the ability to antibacterial performance is controlled by the reaction of cells death[19]. So 2% silver content is selected in order to simultaneously make use of both the effect of powder and the effects of photocatalysis of titanium dioxide and antibacterial performance of Ag+.
4.2 Effect of heating temperature on MiCs
The influence of temperature in heat-treatment on MiCs is shown in Fig.6. Powders obtained at different temperatures have different antibacterial activities. The MiCs of powder decrease slowly with the increase of heating temperature in low temperature range. Here the X-ray diffraction pattern of anatase shows that the peaks of a certain non-crystal phases are broadened and the intensities are quite low. This means that crystal degree of grain is poor, which affects its antibacterial performances[18]. The peaks of X-ray diffraction pattern become narrower and the intensity becomes stronger with the temperature increasing, meaning that the crystal degree improves. So the heat-treatment in the temperature range of 400-600℃ is suitable for the powder. When the temperature is about 600℃ the quick accretion of MiCs and the decline of antibacterial performances result from the fact that the thermochromic reaction of Ag2SO4 takes place by thermal-induction and Ag+ is oxidized to Ag2O[19].
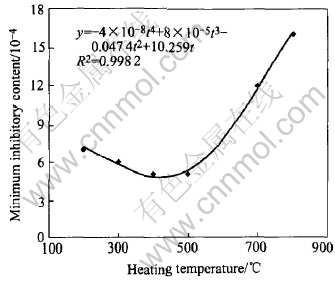
Fig.6 Effect of heating temperature on MiCs
5 CONCLUSIONS
1) When the silver content in titanium dioxide and silver sulfate powder is less than 4%, the MiCs decrease with the increase of silver content. When the silver sulfate is less than 2%, the photocatalysis of titanium dioxide and silver ions cooperate to kill bacilli. At last the MiCs keep around 1.0×10-4-1.5×10-4 constantly with the increase of silver contents.
2) The powder prepared is spherical and the size increases from 10.7nm to 28.7nm as the temperature of heat-treatment increases from 300℃ to 800℃.
3) The MiCs decrease with the temperature increasing when the temperature of heat-treatment is below 500℃, but the antibacterial performance decreases and the MiCs quickly increase when the temperature is beyond 600℃.
REFERENCES
[1]Atsumi K, Sakuma S. Dentifrice Containing Antibacterial Material [P]. Europe Patent, EP0723773,1996-07-31.
[2]Rivera-Garza M, Olguin M T, Garcia-Sosa I, et al. Silver supported on natural Mexican zeolite as an antibacterial material [J]. Microporous and Mesoporous Material, 2000, 39(3): 431-444.
[3]MU Xin-qiang, LI Qi-nian, LIU Xin-nian. Preparation of phosphate glasses antibacterial agents[J]. Journal of Northwest University of Light Industry, 2000, 18(2): 79-82.(in Chinese)
[4]Feng Q L, Wu J, Chen G Q, et al. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus[J]. Biomedical Materials Research, 2000, 52(4): 662-668.
[5]Skmen M, Candan F, Sümer Z. Disinfection of E. coli by the Ag-TiO2/UV system: lipidperoxidation [J]. Journal of Photochemistry and Photobiology, A: Chemistry, 2001, 143(2-3): 241-244.
[6]TANG Bin, ZHANG Qing-qing. The preparation of silver-loaded titanium dioxide film and its photocalalytic property[J]. Photographic Science and Photochemistry, 2003, 21(5): 328-333.(in Chinese)
[7]Kühn K P, Iris C F, Massholder K. Disinfection of surface by the photocatalytic oxidation with titanium dioxide and UVA light [J]. Chemosphere, 2003, 53(1): 71-77.
[8]Yoshinari M, Oda Y, Kato T, et al. Influence of surface modifications to titanium on antibacterial activity in vitro [J]. Biomaterial, 2001, 22(14): 2043-2048.
[9]Li F B, Li X Z. Photocatalytic properties of gold/gold ion-modified titanium dioxide for wastewater treatment[J]. Applied Catalysis A: General, 2002, 228(1-2): 15-27.
[10]Amezaga-Madrid P, Nevarez-Moorillon G V, Orrantia-Borunda E, et al. Photoinduced bactericidal activity against pseudomonas aeruginosa by TiO2 based thin film [J]. FEMS Microbiology Letters, 2002, 211(2): 183-188.
[11]Ghosh K, Maiti S N. Mechanical properties of silver-power-filled polypropylene composites [J]. Journal of Applied Polymer Science, 1996, 60(3): 323-331.
[12]ZU Yong, LI Xiao-e, QU Xiao-guang. Photocatalytic performance and application of nano-titanium dioxide [J]. Advances in Titanium Industry, 1999(2): 23-26.(in Chinese)
[13]Kayano S, Yoshihiko K, Kazubito H, et al. Bactericidal and detoxification effects of TiO2 thin film photocatalysts[J]. Environmental Science and Technology, 1998, 32(5): 726-728.
[14]Byunghoon K, Dohwan K, Donglyum C, et al. Bactericidal effect of TiO2 photocatalyst on selected food-borne pathogenic bacteria [J]. Chemosphere, 2003, 52(1): 277-281.
[15]Kawahara K, Tsuruda K, Morishuita M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic condition[J]. Dental Materials, 2000, 16(6): 452-455.
[16]GAO Lian, ZHENG Shan, ZHANG Qing-hong. Nano-titanium Oxide Mere Catalysis Materials and Applications [M]. Chemical Industry Press, 2002. 15-17.(in Chinese)
[17]Ding Xin-geng, Yang Hui. Fabrication, Antibacterial Properties and Application of Nano-TiO2 Doped with Ag+ [D]. Hangzhou: Zhejiang University, 2001.(in Chinese)
[18]Osamu Y. Infuence of size on the antibacterial activity of zinc oxide [J]. The International Journal of Inorganic Materials, 2001, 3(7): 643-646.
[19]YOU Xiao-zeng. Molecular-based Material—Opto-electronic Functional Compounds [M]. Shanghai: Shanghai Science & Technology Press, 2001. 196.(in Chinese)
Foundation item: Project(04GK2007) supported by Hunan Industrial Key Project of Science and Technology
Received date: 2005-02-28; Accepted date:2005-06-09
Correspondence: PENG Bing, Professor, PhD; Tel: +86-731-8830875; E-mail:Pengyu@public.cs.hn.cn


