Trans. Nonferrous Met. Soc. China 27(2017) 2481-2491
Biological treatment of wastewater with high concentrations of zinc and sulfate ions from zinc pyrithione synthesis
Zhi-xiong PENG1,2, Hui-jun HE1,2, Chun-ping YANG1,2,3, Guang-ming ZENG1,2, Shan WEN1,2, Zhou YAN1,2, Hai-hong XIANG1,2, Yan CHENG1,2, Sheldon TARRE4, Michal GREEN4
1. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China;
2. Key Laboratory of Environmental Biology and Pollution Control (Hunan University), Ministry of Education, Changsha 410082, China;
3. Zhejiang Provincial Key Laboratory of Solid Waste Treatment and Recycling, College of Environmental Science and Engineering, Zhejiang Gongshang University, Hangzhou 310018, China;
4. Faculty of Civil and Environmental Engineering, Israel Institute of Technology, Haifa 32000, Israel
Received 24 July 2016; accepted 24 March 2017
Abstract:
An enriched and domesticated bacteria consortium of sulfate-reducing bacteria (SRB) was used to treat wastewater from zinc pyrithione (ZPT) production, and the effects of different reaction parameters on sulfate reduction and zinc precipitation were evaluated. The single-factor experimental results showed that the removal rates of Zn2+ and 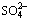 decreased with an increased ZPT concentration ranging from 3.0 to 5.0 mg/L. Zn2+ and
decreased with an increased ZPT concentration ranging from 3.0 to 5.0 mg/L. Zn2+ and 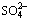 in wastewater were effectively removed under the conditions of 30-35 °C, pH 7-8 and an inoculum concentration of 10%-25%. The presence of Fe0 in the SRB system enhanced Zn2+ and
in wastewater were effectively removed under the conditions of 30-35 °C, pH 7-8 and an inoculum concentration of 10%-25%. The presence of Fe0 in the SRB system enhanced Zn2+ and 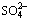 removal and may increase the resistance of SRB to the toxicity of Zn2+ and ZPT in wastewater. A Box-Behnken design was used to evaluate the influence of the main operating parameters on the removal rate of
removal and may increase the resistance of SRB to the toxicity of Zn2+ and ZPT in wastewater. A Box-Behnken design was used to evaluate the influence of the main operating parameters on the removal rate of 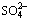 . The optimum parameter values were found to be pH 7.45, 33.61 °C and ZPT concentration of 0.62 mg/L, and the removal rate of
. The optimum parameter values were found to be pH 7.45, 33.61 °C and ZPT concentration of 0.62 mg/L, and the removal rate of 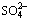 reached a maximum of 91.62% under these optimum conditions.
reached a maximum of 91.62% under these optimum conditions.
Key words:
biological treatment; sulfate reduction; sulfate-reducing bacteria; wastewater; zinc pyrithione; zinc;
1 Introduction
Zinc pyrithione (ZPT) is a broad-spectrum anti- bacterial agent and extensively used as the active antidandruff ingredient in hair care. ZPT is an extremely toxic substance whose EC50 value was found to be less than 1 mg/L for Vibrio fischeri, Pseudomonas putida and Tetrahymena thermophila, and some of the main microorganisms present in activated sludge [1]. In seawater, it was found that ZPT exposed to the sunlight degrades rapidly (half-life <2 min) into less toxic compounds, and so it is presumed to be environmentally neutral and non-persistent in the aquatic environment. However, other studies have reported that half of the initial ZPT amount remained after 48 h light exposure. Therefore, biological treatment processes for ZPT wastewater could be adversely affected if ZPT production wastewater is directly discharged without proper pretreatment.
ZPT production wastewater contains high concentrations of Zn2+ and 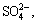 increasing the difficulties of ZPT production wastewater treatment. Generally, most common zinc salts at high concentrations can be separated effectively by physiochemical methods [2-4]. However, this implies prohibitively high operative cost and causes secondary pollution when applied on large scale. Bacterial sulfate reduction is considered to be a highly promising method for the removal of sulfate and heavy metal from environmental contamination due to low operating cost, high selectivity, complete removal and only small residual sludge. Anaerobic sulfate reducing bacteria (SRB) could oxidize various organic compounds by utilizing sulfate as an electron acceptor, resulting in alkalinity generation and metal cation (Me2+) precipitation as sulphide, which is conducive to the sustainable development of the ZPT production industry.
increasing the difficulties of ZPT production wastewater treatment. Generally, most common zinc salts at high concentrations can be separated effectively by physiochemical methods [2-4]. However, this implies prohibitively high operative cost and causes secondary pollution when applied on large scale. Bacterial sulfate reduction is considered to be a highly promising method for the removal of sulfate and heavy metal from environmental contamination due to low operating cost, high selectivity, complete removal and only small residual sludge. Anaerobic sulfate reducing bacteria (SRB) could oxidize various organic compounds by utilizing sulfate as an electron acceptor, resulting in alkalinity generation and metal cation (Me2+) precipitation as sulphide, which is conducive to the sustainable development of the ZPT production industry.
 +2CH2O+2H+→H2S+2H2CO3 (1)
+2CH2O+2H+→H2S+2H2CO3 (1)
Me2++H2S→MeS↓+2H+ (2)
Therefore, the immobilization removal of Zn2+ through microbial mediated reduction and precipitation has attracted a great deal of interest [5,6]. The tolerance concentration of SRB to Zn2+ depends on the type of SRB. AZABOU et al [7] found that zinc concentration more than 150 mg/L was lethal to SRB. ZHOU et al [8] reported that Zn2+ inhibited SRB when the concentration of Zn2+ was 300 mg/L, while the tolerance of SRB to Zn2+ could be enhanced to some extent by acclimatization. Although there are many reports on SRB and metal precipitation as mentioned above, literature on cost-effective processes for simultaneous removal of Zn2+ and  from wastewater in the presence of toxic ZPT is still not available. ZPT can be dead for SRB, which can lead to unstable biological treatment operation or even system failure. Most importantly, the concentration of zinc sulfate in ZPT production wastewater can reach 1000 mg/L that is harmful to SRB [9,10]. To address this problem, Fe0 was introduced into the processing stream and found to be beneficial for forming an anaerobic reducing environment. A synergetic enhancement was also reported when Fe0 was applied to the treatment of uranium-bearing wastewater [11]. Thus, it is reasonable to hypothesize that Fe0 might promote Zn2+ removal as well [8,12].
from wastewater in the presence of toxic ZPT is still not available. ZPT can be dead for SRB, which can lead to unstable biological treatment operation or even system failure. Most importantly, the concentration of zinc sulfate in ZPT production wastewater can reach 1000 mg/L that is harmful to SRB [9,10]. To address this problem, Fe0 was introduced into the processing stream and found to be beneficial for forming an anaerobic reducing environment. A synergetic enhancement was also reported when Fe0 was applied to the treatment of uranium-bearing wastewater [11]. Thus, it is reasonable to hypothesize that Fe0 might promote Zn2+ removal as well [8,12].
The objective of this study was to investigate the effects of different experimental parameters on Zn2+ and  removal under different ZPT concentrations together with the growth dynamics of SRB. Batch experiments were performed and parameters including Zn2+ concentration, pH, inoculum concentration, temperature, and ZPT concentrations from 1.0 to 5.0 mg/L were examined. The reduction of Zn2+ and
removal under different ZPT concentrations together with the growth dynamics of SRB. Batch experiments were performed and parameters including Zn2+ concentration, pH, inoculum concentration, temperature, and ZPT concentrations from 1.0 to 5.0 mg/L were examined. The reduction of Zn2+ and  over time in the combined system of SRB and Fe0 (SRB+Fe0) was also investigated to study the feasibility of SRB+Fe0 in ZPT wastewater treatment. In addition, a Box-Behnken design was selected to determine the optimum conditions for ZPT production wastewater treatment and to illustrate the relationship between sulfate removal and three independent variables including temperature, pH, and initial ZPT concentration.
over time in the combined system of SRB and Fe0 (SRB+Fe0) was also investigated to study the feasibility of SRB+Fe0 in ZPT wastewater treatment. In addition, a Box-Behnken design was selected to determine the optimum conditions for ZPT production wastewater treatment and to illustrate the relationship between sulfate removal and three independent variables including temperature, pH, and initial ZPT concentration.
2 Experimental
2.1 Chemicals
ZPT, purity above 96%, was purchased from Shanghai DIBO Chemical Technology Co., Ltd. Zinc standard solution (1.0 mg/mL) was supplied by Beijing PUXI Technology Co., Ltd. All other chemicals and reagents used in this study were of analytical grade.
2.2 Preparation of SRB for reduction
A mixed SRB consortium was isolated from landfill leachate collected from a landfill in Hunan Province, China. The landfill leachate was transferred immediately to a 250 mL serum bottle until it was completely filled, then the bottle was sealed tightly to prevent direct contact with atmospheric oxygen. After that, the sample was transported to a laboratory in a cool box. Once in the laboratory, the sample was removed from the cool box. The sample was purged with high purity nitrogen to minimize the exposure of SRB to oxygen.
To a 250 mL serum bottle, 25 mL of landfill leachate was added followed by 225 mL of sterilized Postgate’s B medium [13] (autoclaved at 121 °C and 2.026×105 Pa for 20 min) at pH 7.5. After purging with pure nitrogen for 10 min, the bottle was capped with a tertbutyl rubber stopper and crimp-sealed, then incubated for at 35 °C 4 d in an incubator. Enriched SRB culture was obtained by repeating the process mentioned above three times. Postgate’s B medium contained 3.5 g/L sodium lactate, 1.0 g/L yeast extract, 2.0 g/L MgSO4·7H2O, 0.5 g/L Na2SO4, 0.5 g/L K2HPO4, 1.0 g/L NH4Cl, 0.5 g/L FeSO4·7H2O, 0.1 g/L ascorbic acid, and 0.1 g/L of thioglycolic acid.
The SRB was acclimated to Zn2+ tolerance in the following manner. After enrichment, 25 mL SRB was inoculated into 250 mL serum bottle containing 225 mL sterilized Postgate’s B medium at pH 7.5 and amended with approximately 50 mg/L Zn2+. The headspace of serum bottle was filled with pure N2 before being capped and crimp-sealed. The bottle was maintained in an incubator at 35 °C for 4 d. This procedure was repeated until the medium turned black within 2 d. The zinc concentration in the Postgate’s B medium was increased at a step of 50 mg/L from 100 to 350 mg/L. Once the acclimated SRBs were all prepared, they were streaked on agar slants spiked with Zn2+, incubated at 35 °C for 4 d and stored at 4 °C for use.
2.3 Analytical methods
All glassware and plasticware used were thoroughly cleaned by soaking in 10% nitric acid for 48 h followed by ultrasonic cleaning for 30 min, rinsed several times with ultrapure water and finally oven dried. Before the measurement of soluble Zn and sulfate, samples were centrifuged at 4000 r/min for 10 min. The supernatant was analyzed for Zn2+ by atomic absorption spectroscopy (Perkin Elmer Analyst 700). 5.0 mL of the supernatant was transferred to 50 mL volumetric flasks and diluted with 0.1 mol/L HNO3 to dissolve Zn particles. Diluted samples were then filtered through a sterile nitrocellulose filter (0.45 mm) to remove biomass and other particles before analysis.
Barium chromate spectrophotometry was used for determination of sulfate by visible spectrophotometer (Shanghai No. 3 Analytical Instruments Company). Sulfate was measured immediately after centrifugation mentioned above. Each experiment was carried out in triplicate under identical condition.
The growth curves of SRB under different concentrations of ZPT were studied using nephelometery by visible range spectrophotometer. ZPT was added into the modified Postgate’s B medium according to the concentrations of 0, 1.0, 3.0 and 5.0 mg/L, respectively. 1.0 mL of acclimated SRB and 9.0 mL of sterilized Postgate’s B medium containing ZPT (pH 7.5) were transferred into 10 mL sterile centrifuge tube. The centrifuge tubes were put into an incubator at 35 °C for 50 h, and the peak absorbance at 600 nm (OD600)was monitored every 2 h. The SRB concentration showed a linear relationship with OD600 as follows:
c=4.6×107OD600, R2=0.990 (3)
where c is the SRB concentration (cfu/mL).
2.4 Batch studies
All experiments for Zn2+ removal and  reduction were performed in duplicate 250 mL serum bottles, capped with tertbutyl rubber stopper and crimp- sealed. 25 mL SRB, a given quantity of ZnSO4 and 225 mL sterile Postgate’s B medium at pH 7.0 were added into 250 mL serum bottle. In order to estimate and assess the potential toxicity that ZPT could cause on SRB, ZPT was added into the bottles at a concentration of 0, 1.0, 3.0 and 5.0 mg/L, respectively. The bottles were put into an incubator at 30 °C for 7 d. The residual concentration of Zn2+ was measured every day at various initial concentrations of 50, 100, 150, 200, 250, 300 and 350 mg/L. The solution pH was adjusted to the desired values (5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5 and 9.0) by 2 mol/L NaOH and HCl to evaluate the effect of pH on precipitation of Zn2+ and degradation of
reduction were performed in duplicate 250 mL serum bottles, capped with tertbutyl rubber stopper and crimp- sealed. 25 mL SRB, a given quantity of ZnSO4 and 225 mL sterile Postgate’s B medium at pH 7.0 were added into 250 mL serum bottle. In order to estimate and assess the potential toxicity that ZPT could cause on SRB, ZPT was added into the bottles at a concentration of 0, 1.0, 3.0 and 5.0 mg/L, respectively. The bottles were put into an incubator at 30 °C for 7 d. The residual concentration of Zn2+ was measured every day at various initial concentrations of 50, 100, 150, 200, 250, 300 and 350 mg/L. The solution pH was adjusted to the desired values (5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5 and 9.0) by 2 mol/L NaOH and HCl to evaluate the effect of pH on precipitation of Zn2+ and degradation of  . The influence of temperature on the removal of Zn2+ and
. The influence of temperature on the removal of Zn2+ and  was examined at 15, 20, 25, 30, 35 and 40 °C. The effect of Fe0 on removal of Zn2+ and
was examined at 15, 20, 25, 30, 35 and 40 °C. The effect of Fe0 on removal of Zn2+ and  was investigated by measuring the residual Zn2+ and
was investigated by measuring the residual Zn2+ and  each day for 7 d.
each day for 7 d.
2.5 Experimental design
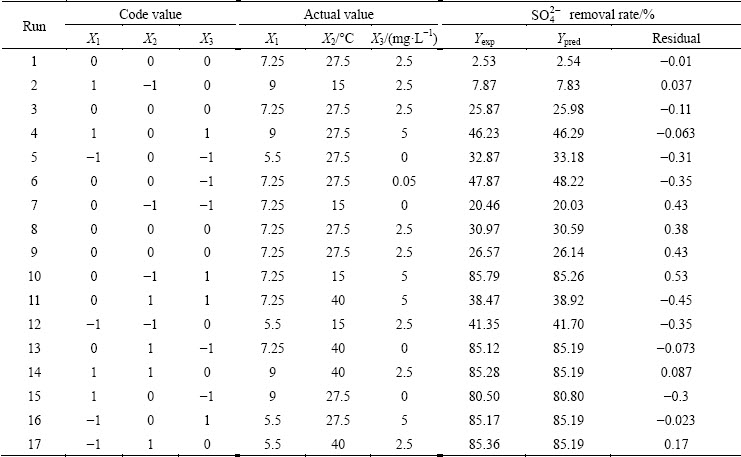
The sulfate removal rate was optimized by response surface methodology (RSM) using a Box–Behnken design (BBD). The statistical software Design Expert 8.0.6 was used for the analysis. Three independent parameters, pH, X1 (5.5-9), temperature, X2 (15-40 °C) and ZPT concentration, X3 (1-5 mg/L) were confirmed to optimize the sulfate removal. The coded and uncoded levels of these variables were presented in Table 1. With statistical analysis of the gained experimental data, a quadratic equation (Eq. (4)) was attained as an empirical model for the optimization process.
Table 1 Box–Behnken design matrix
Y=β0+∑βixi++∑βiixi2+∑βijxij (4)
where Y is the response, β0, βi, βii, and βij are coefficients of the intercept, linear, square and interaction effects, respectively. The optimum response (Yopt) and the corresponding process parameters were also determined.
3 Results and discussion
3.1 Effect of ZPT concentration on growth of SRB
As an antimicrobial agent, ZPT is active against a range of micro-organisms. The growth characteristics of SRB under ZPT concentrations of 0, 1.0, 3.0 and 5.0 mg/L in 50 h batch tests were investigated (Fig. 1). All the curves illustrate the characteristic growth phase in SRB batch culture. From Fig. 1, it can be seen that the concentration of SRB decreased sharply within 8 h after inoculation for all tests. The SRB biomass reached a minimum at 8 h. After a period of acclimatization, SRB entered logarithmic growth phase. Although the logarithmic growth phase of SRB under different concentrations of ZPT was similar, its concentration decreased with the increase of ZPT concentrations at the end of logarithmic growth phase. In culture medium with ZPT, SRB showed poor growth and entered into aging phase much earlier as compared to SRB in medium without ZPT. In addition, the concentration of SRB decreased rapidly during aging phrase with ZPT, while the concentration of SRB showed only a slight drop in the absence of ZPT. Moreover, the concentration of SRB with ZPT was significantly lower than that without ZPT when the trials ended.
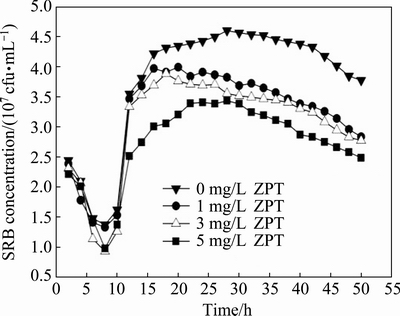
Fig. 1 Growth curves for SRB grown under four ZPT concentration levels within 50 h
During the aging phase, there was no increase in the concentration of cells for the ZPT-free group and growth was limited by insufficient nutrients and the accumulation of cellular metabolism by-products [14]. Possibly, the nutrient limitations and the accumulation of high concentrations of sulfide produced during the exponential phase hindered the growth of SRB. It was suggested that high sulfide concentration has a reversible and direct toxicity effect on SRB. The inhibition may be the result of an intrinsic toxicity of H2S to living systems or it may be a result of indirect toxicity generated by rendering the iron insoluble as iron sulfide [15]. Iron is needed as an essential cofactor to various cytochromes involved in cellular respiration (e.g., cytochrome-C). In addition to the negative effects of insufficient nutrients and H2S, ZPT inhibited the growth of SRB in the ZPT-containing batch experiments. ZPT inhibited the growth and metabolism of SRB, and the extent of inhibition increased with increasing ZPT concentration, with the toxicity especially strong when the ZPT concentration was 5.0 mg/L. Similarly, ZPT was shown to inhibit yeast growth at concentrations lower than 1 mg/L through copper influx and inactivation of iron- sulfur proteins. Perhaps, this was because ZPT was toxic for SRB.
3.2 Effects of initial Zn2+ concentration on removal of Zn2+
Although Zn2+ in trace concentration is beneficial to the growth of SRB, it inhibits its growth at high concentrations by interfering with nucleic acids and active sites of enzymes [16,17]. The effects of the initial concentration of Zn2+ on the removal of Zn2+ are presented in Fig. 2. From Fig. 2, it can be seen that the removal rate of Zn2+ gradually decreased with an increased concentration of Zn2+, and exceeded 85.0% when the initial Zn2+ concentration was lower than 250 mg/L. When the initial Zn2+ concentration was higher than 250 mg/L, a much more dramatic drop of Zn2+ removal rate was observed and displayed a severe toxic effect on the growth of SRB. The results showed that 250 mg/L was the maximum tolerable concentration of Zn2+ for SRB, higher than 150 mg/L obtained in an earlier study [18]. It should be noted that not only high concentration of heavy mental but insoluble metal sulfides can inhibit the SRB activity as well [19]. Mental sulfides concentrated near the bacteria cells or deposited on the surface of the SRB hindered further metabolism by preventing the contact between the necessary enzymes and reactants. The toxicity of the combination of ZPT and Zn2+ on SRB was marginally higher than that of Zn2+ only.
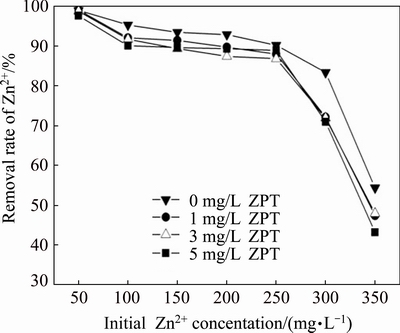
Fig. 2 Effect of initial Zn2+ concentration on Zn2+ removal rate by SRB consortium at four different concentrations of ZPT
3.3 Effect of pH on removal of Zn2+ and 
The optimum pH for growth of SRB is pH 5.5 to 9 [20]. pH plays an important role in the biogenic sulfide production which in turn affects the removal of Zn2+ and  [8]. The results are presented in Fig. 3. There were clear differences between the removal of Zn2+ and
[8]. The results are presented in Fig. 3. There were clear differences between the removal of Zn2+ and  . When the concentration of ZPT was greater than 1.0 mg/L and the pH was lower than 7.0 or higher than 8.0, the removal rate of
. When the concentration of ZPT was greater than 1.0 mg/L and the pH was lower than 7.0 or higher than 8.0, the removal rate of  decreased substantially while Zn2+ could be efficiently removed at pH 6.5-9.0. This occurred because the solubility of Zn2+ is pH-dependent, while at higher pH zinc is less soluble in water. In addition, the bioavailability of heavy metals is critically dependent on its speciation, and it is generally assumed that free metal ions are most toxic to microorganisms [21]. Therefore, the effects mentioned above facilitate the removal of Zn2+ while in contrast the removal rate of
decreased substantially while Zn2+ could be efficiently removed at pH 6.5-9.0. This occurred because the solubility of Zn2+ is pH-dependent, while at higher pH zinc is less soluble in water. In addition, the bioavailability of heavy metals is critically dependent on its speciation, and it is generally assumed that free metal ions are most toxic to microorganisms [21]. Therefore, the effects mentioned above facilitate the removal of Zn2+ while in contrast the removal rate of  is low in alkaline conditions.
is low in alkaline conditions.
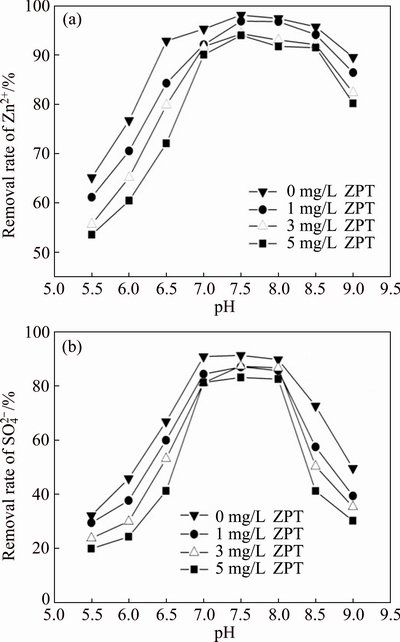
Fig. 3 Effect of pH on Zn2+ (a) and  (b) removal rates by SRB consortium at four different concentrations of ZPT and initial Zn2+ concentration of 100 mg/L
(b) removal rates by SRB consortium at four different concentrations of ZPT and initial Zn2+ concentration of 100 mg/L
The removal rate of  is an important indicator for the activity of SRB. From Fig. 3(b) both acidic and basic conditions pose a significant barrier for the growth of SRB. In addition, when the culture was not given as ZPT, the removal rates of Zn2+ and
is an important indicator for the activity of SRB. From Fig. 3(b) both acidic and basic conditions pose a significant barrier for the growth of SRB. In addition, when the culture was not given as ZPT, the removal rates of Zn2+ and  are higher than those cultured with ZPT addition, especially for
are higher than those cultured with ZPT addition, especially for  . These results might be attributed to the inhibition effect of ZPT on SRB being enhanced in acidic and alkaline conditions. The removal rates of Zn2+ and
. These results might be attributed to the inhibition effect of ZPT on SRB being enhanced in acidic and alkaline conditions. The removal rates of Zn2+ and  are the highest in the pH range from 7.0 to 8.0, with the highest at pH 7.5. In this range, ZPT had little effect on the SRB.
are the highest in the pH range from 7.0 to 8.0, with the highest at pH 7.5. In this range, ZPT had little effect on the SRB.
3.4 Effect of temperature on removal of Zn2+ and 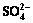
The effects of temperature on removal rates of Zn2+ and  are presented in Fig. 4. From Fig. 4, it can be seen that the removal rates of Zn2+ and
are presented in Fig. 4. From Fig. 4, it can be seen that the removal rates of Zn2+ and  increased with increasing temperature until 35 °C. The removal rates of Zn2+ and
increased with increasing temperature until 35 °C. The removal rates of Zn2+ and  with ZPT were lower than that of the ZPT-free group and decreased with an increase of ZPT concentration within the temperature range of 20-40 °C. The removal rates of Zn2+ and
with ZPT were lower than that of the ZPT-free group and decreased with an increase of ZPT concentration within the temperature range of 20-40 °C. The removal rates of Zn2+ and  in all of the trials reached a maximum value at 35 °C.
in all of the trials reached a maximum value at 35 °C.
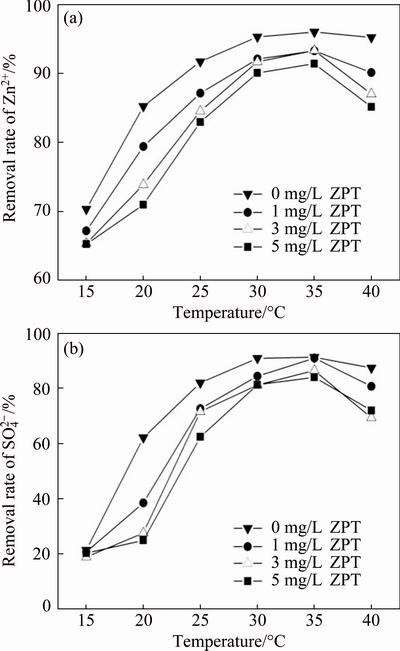
Fig. 4 Effect of temperature on Zn2+ (a) and 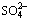 (b) removal rates by SRB consortium at four different concentrations of ZPT and initial Zn2+ concentration of 100 mg/L
(b) removal rates by SRB consortium at four different concentrations of ZPT and initial Zn2+ concentration of 100 mg/L
As an important factor in biological and chemical reduction [8], temperature may provide insight into the mechanism of the interaction between SRB and ZPT, which could provide new significant information to promote technological advance in practical application.  reduction is highly sensitive to temperature change with effective reduction occurring at 30-40 °C, while Zn2+ reduction has a much wider temperature range (20-40 °C). As 35 °C is the most suitable temperature for SRB reduction reaction, a temperature increase or decrease suppresses these reactions [8]. When ambient temperatures were as low as 15 °C, the activity of sulfate reducing bacteria was significantly inhibited. It may be caused by the decrease in enzyme activity related to sulfate reduction and the extension of the acclimation period [22]. The experiment results also show that the toxicity of the ZPT on SRB is reduced within the favorable temperature range of 30-35 °C.
reduction is highly sensitive to temperature change with effective reduction occurring at 30-40 °C, while Zn2+ reduction has a much wider temperature range (20-40 °C). As 35 °C is the most suitable temperature for SRB reduction reaction, a temperature increase or decrease suppresses these reactions [8]. When ambient temperatures were as low as 15 °C, the activity of sulfate reducing bacteria was significantly inhibited. It may be caused by the decrease in enzyme activity related to sulfate reduction and the extension of the acclimation period [22]. The experiment results also show that the toxicity of the ZPT on SRB is reduced within the favorable temperature range of 30-35 °C.
3.5 Effect of inoculum concentration on removal of Zn2+ and 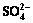
Regarding the effect of inoculum concentration, the common assumption is that excessive cell density leads to a decrease in the substrate/microorganism (S/M) ratio, causing a shortage of substrate to support microbial metabolism. However, low inoculum concentration may result in the death of bacteria. Therefore, the suitable inoculum concentration could shorten the time of the acclimatization period, which in turn affects the removal of heavy metals [23].
The effect of inoculum concentration on the removal rates of Zn2+ and  is presented in Fig. 5. When the inoculum concentration was 5%, the removal rates of Zn2+ and
is presented in Fig. 5. When the inoculum concentration was 5%, the removal rates of Zn2+ and  were much more sensitive to the addition of ZPT. In contrast, the removal rates of Zn2+ and
were much more sensitive to the addition of ZPT. In contrast, the removal rates of Zn2+ and  significantly increased for SRB exposed to ZPT when the inoculum concentration increased from 5% to 10%. As seen from Fig. 5(b), when the inoculum concentration was 10%, the removal rate of
significantly increased for SRB exposed to ZPT when the inoculum concentration increased from 5% to 10%. As seen from Fig. 5(b), when the inoculum concentration was 10%, the removal rate of  was between 81.2%-90.8% with and without ZPT. In comparison, at a 5% inoculation level, the range of
was between 81.2%-90.8% with and without ZPT. In comparison, at a 5% inoculation level, the range of  removal rate was much lower between 30%-59% at ZPT concentrations of 1.0, 3.0 and 5.0 mg/L. Inoculum concentrations higher than 10% showed little improvement in the removal rates of Zn2+ and
removal rate was much lower between 30%-59% at ZPT concentrations of 1.0, 3.0 and 5.0 mg/L. Inoculum concentrations higher than 10% showed little improvement in the removal rates of Zn2+ and  .
.
The reasons might be the high toxicity of ZPT to SRB. When the SRB inoculum concentration was low at 5%, ZPT inhibited SRB activity and inactivated most of the bacteria, making it difficult for SRB to multiply rapidly. Low ZPT concentrations (1 mg/L) showed low toxicity to SRB, while strong inhibition was observed at high concentrations of ZPT. As seen in Fig. 5(b), 3.0-5.0 mg/L ZPT significantly inhibited the growth of SRB at an inoculum concentration of 5%. However, 3.0-5.0 mg/L ZPT was tolerable to SRB when the inoculum concentration was more than 10%.
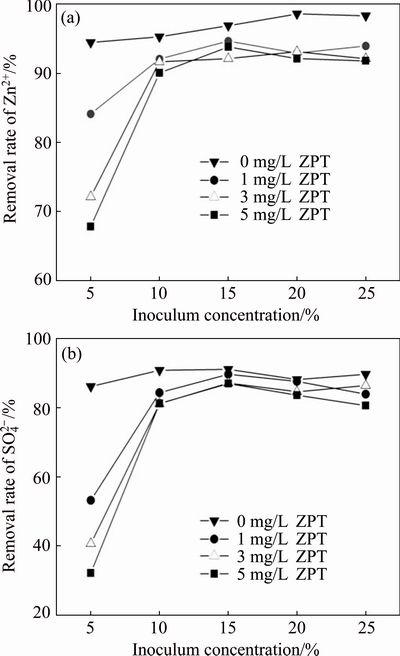
Fig. 5 Effect of inoculum concentration on Zn2+ (a) and  (b) removal rates by SRB consortium at four different concentrations of ZPT and initial Zn2+ concentration of 100 mg/L
(b) removal rates by SRB consortium at four different concentrations of ZPT and initial Zn2+ concentration of 100 mg/L
3.6 Removal of Zn2+ and  in systems using SRB+Fe0
in systems using SRB+Fe0
The removal rates of Zn2+ and  in different systems were investigated over time and the results are shown in Fig. 6. In SRB system, the residual Zn2+ concentration showed a consistent pattern of the first up and and then down fluctuation. This fluctuation increased with an increased ZPT concentration. However, there was no evident fluctuation of Zn2+ removal in SRB+Fe0 system.
in different systems were investigated over time and the results are shown in Fig. 6. In SRB system, the residual Zn2+ concentration showed a consistent pattern of the first up and and then down fluctuation. This fluctuation increased with an increased ZPT concentration. However, there was no evident fluctuation of Zn2+ removal in SRB+Fe0 system.  removal rates in SRB+Fe0 system and SRB system without ZPT reached more than 90% even on the 5th day after inoculation, and the removal rates on the 7th day could reach 91.9% and 90.8%, respectively. In contrast,
removal rates in SRB+Fe0 system and SRB system without ZPT reached more than 90% even on the 5th day after inoculation, and the removal rates on the 7th day could reach 91.9% and 90.8%, respectively. In contrast,  removal rate with ZPT was stably increasing until the end of experiment, correspondingly arriving at 84.4%, 81.2% and 81.2% under the ZPT concentrations of 0, 1.0, 3.0 and 5.0 mg/L. From Fig. 6(b), it can be seen that the consumption rate of
removal rate with ZPT was stably increasing until the end of experiment, correspondingly arriving at 84.4%, 81.2% and 81.2% under the ZPT concentrations of 0, 1.0, 3.0 and 5.0 mg/L. From Fig. 6(b), it can be seen that the consumption rate of  in this study decreased as the ZPT concentration increased. Comparing three concentrations of ZPT, the final removal rates of
in this study decreased as the ZPT concentration increased. Comparing three concentrations of ZPT, the final removal rates of  had no significant difference on the 7th day, which were much lower than that of the system without ZPT.
had no significant difference on the 7th day, which were much lower than that of the system without ZPT.
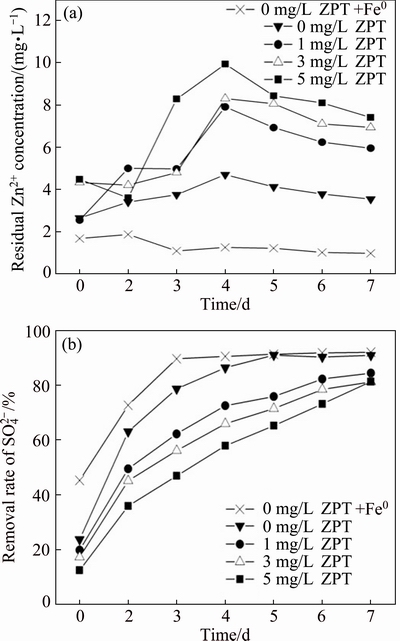
Fig. 6 Variations of residual Zn2+ concentration (a) and 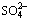 removal rate (b) with time under four different concentrations of ZPT in different systems at initial Zn2+ concentration of 100 mg/L
removal rate (b) with time under four different concentrations of ZPT in different systems at initial Zn2+ concentration of 100 mg/L
The mechanisms of zinc removal in SRB system were sulfide precipitation and integral components of biosorption [24]. Biodegradation was the major mechanism of Zn2+ removal on the first day since the removal rate of  on the first day was very low and the residual Zn2+ concentration increased until the 4th day. The reason for the decrease in removal rate of Zn2+ within 4 d was that the activity of SRB was constrained seriously by ZPT and ZnS, which weakened the effect of biosorption. The mechanisms of Zn2+ removal in SRB+Fe0 system were rather complex, including sulfide precipitation, biosorption and reductive precipitation [25].
on the first day was very low and the residual Zn2+ concentration increased until the 4th day. The reason for the decrease in removal rate of Zn2+ within 4 d was that the activity of SRB was constrained seriously by ZPT and ZnS, which weakened the effect of biosorption. The mechanisms of Zn2+ removal in SRB+Fe0 system were rather complex, including sulfide precipitation, biosorption and reductive precipitation [25].
3.7 Optimization by Box–Behnken design
The Box–Behnken design was applied in this study and 17-experimental runs were conducted at orders randomly for the optimization of sulfate removal. The effects of key parameters, pH (X1), temperature (X2) and ZPT concentration (X3) on sulfate production were evaluated. According to the RSM results in regard to the response variables of sulfate removal rate, which were acquired from 17 groups of experiments with the help of Design-Expert software, regression analysis of data from Table 2 resulted in the following quadratic expression (see Eq. (5)):
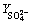 =85.19+6.40X1+15.48X2-7.69X3+3.75X1X2-1.12X2X3-14.08X1X3-39.77X12-24.76X22-12.42X32 (5)
=85.19+6.40X1+15.48X2-7.69X3+3.75X1X2-1.12X2X3-14.08X1X3-39.77X12-24.76X22-12.42X32 (5)
The results of analysis of variance (ANOVA) are listed in Table 2. Statistical testing was carried out by the calculated Fischer values (F-test) and probability values (p-value). The corresponding parameter is more significant if its p-value is smaller than 0.05 at 95% confidence level [26]. Obviously, as shown in Table 2, the model F-value of 7339.99 and values of “p-value>F” less than 0.0001 indicated that the model was significant. There was only a 0.0089% chance that a large “Model F-value” could occur due to noise. The “lack of Fit F-value” of 31.11 implied that the lack of fit was significant. In this study, the independent variables of the quadratic model pH X1, temperature X2 and ZPT concentration X3, the interaction between pH and temperature (X1X2), the interaction between pH and ZPT concentration (X1X3) and the interaction between temperature and ZPT concentration (X2X3) were quite significant because the p-value was less than 0.05. Judging by the F-values of the items in the regression model, the temperature (X2) had the highest F-value (9000.28) with the lowest p-value (<0.0001) among other parameters, so the degree of importance of the three parameters on  removal rate was: temperature X2>ZPT concentration X3>pH X1. The quite high R2 values of 0.9999 for
removal rate was: temperature X2>ZPT concentration X3>pH X1. The quite high R2 values of 0.9999 for  removal rate indicated that the predicted polynomial model was reasonably well fitted with the data. The predicted R2 (Pred) values of 0.9998 for sulfate removal rate were in reasonable agreement with the adjusted R2 (Adj) values of 0.9987 for
removal rate indicated that the predicted polynomial model was reasonably well fitted with the data. The predicted R2 (Pred) values of 0.9998 for sulfate removal rate were in reasonable agreement with the adjusted R2 (Adj) values of 0.9987 for  removal rate. The comparisons between experimental and predicted values of
removal rate. The comparisons between experimental and predicted values of  removal rate were exhibited graphically with 45 °C-lines in Fig. 7. Very little deviation was discovered between points that represented experimental values and the regression line that represented predicted values, indicated a satisfactory degree of precision and reliability of the experimental values.
removal rate were exhibited graphically with 45 °C-lines in Fig. 7. Very little deviation was discovered between points that represented experimental values and the regression line that represented predicted values, indicated a satisfactory degree of precision and reliability of the experimental values.
Figure 8(a) clearly represented the effects of pH (X1) and temperature (X2) on the sulfate removal rate, when the ZPT concentration (X3) was fixed at 2.5 mg/L. The three-dimensional response surface plot suggests that the interaction effect of pH and temperature signifi- cantly influenced sulfide production (p-value <0.0001). At the lower pH, sulfate removal rate decreased gradually at the lower or higher of the temperature. In addition, when a high level of temperature was applied (40 °C), the removal rate of  was found to increase and then decline by increasing pH. Temperature and pH were key factors influencing the efficiency of sulfide production, which could affect the activity of the enzyme in the reaction of sulfate reduction [22]. However, the
was found to increase and then decline by increasing pH. Temperature and pH were key factors influencing the efficiency of sulfide production, which could affect the activity of the enzyme in the reaction of sulfate reduction [22]. However, the  removal rate was more sensitive to temperature. Figure 8(b) represents response surface plot of two variables pH (X1) and ZPT concentration (X3) while temperature (X2) was kept constant at 27.5 °C. With pH at low levels,
removal rate was more sensitive to temperature. Figure 8(b) represents response surface plot of two variables pH (X1) and ZPT concentration (X3) while temperature (X2) was kept constant at 27.5 °C. With pH at low levels,  removal rate was higher with the decrease of the ZPT concentration owning to the increase of toxicity of ZPT in high ZPT concentration. On the other hand, with pH at high levels, the
removal rate was higher with the decrease of the ZPT concentration owning to the increase of toxicity of ZPT in high ZPT concentration. On the other hand, with pH at high levels, the  removal rate was found to increase first followed by a slight decline with the increasing pH. That was probably because enzyme activity was suppressed under acid-base environment. The result is consistent with AHMADI et al [27], who suggested that there was a strong correlation between the
removal rate was found to increase first followed by a slight decline with the increasing pH. That was probably because enzyme activity was suppressed under acid-base environment. The result is consistent with AHMADI et al [27], who suggested that there was a strong correlation between the  removal rate and the pH. The lower the
removal rate and the pH. The lower the  removal rate was, the lower the observed solution pH was. PIKUTA et al [28] also reported that the reduction of sulfate was usually suppressed at pH values lower than 6 or higher than 9. When the pH value was below 6, the production of the undissociated form (H2S) was a strong inhibitor of SRB [29].
removal rate was, the lower the observed solution pH was. PIKUTA et al [28] also reported that the reduction of sulfate was usually suppressed at pH values lower than 6 or higher than 9. When the pH value was below 6, the production of the undissociated form (H2S) was a strong inhibitor of SRB [29].
Table 2 Analysis of variance (ANOVA) for sulfate removal rate
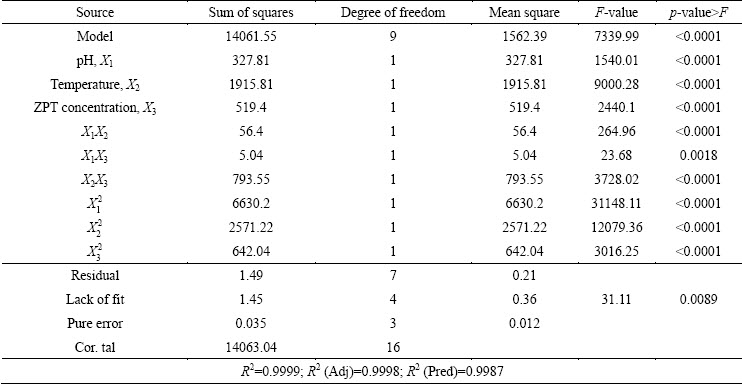
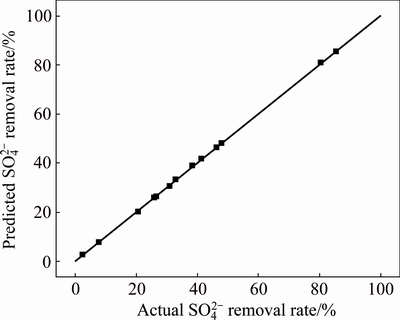
Fig. 7 Comparison of experimental 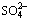 removal rate with calculated one via Box–Behnken design (BBD) resulted equation
removal rate with calculated one via Box–Behnken design (BBD) resulted equation
Figure 8(c) illustrated the effect of temperature (X2) and ZPT concentration (X3) on sulfate removal rate at pH value of 7.25.  removal rate was sensitive to changes in temperature and ZPT concentration, which has the greatest significant interaction on sulfide production (F-value=3728.02, p-value <0.0001). It was obvious that the
removal rate was sensitive to changes in temperature and ZPT concentration, which has the greatest significant interaction on sulfide production (F-value=3728.02, p-value <0.0001). It was obvious that the  removal rate decreased rapidly with the increase of ZPT concentration under low temperature condition, while the
removal rate decreased rapidly with the increase of ZPT concentration under low temperature condition, while the  removal rate decreased slightly under high temperature condition. It may be because the toxicity of ZPT to SRB was weakened under the high temperature condition. Most importantly, all these results are consistent with the single factor experiment.
removal rate decreased slightly under high temperature condition. It may be because the toxicity of ZPT to SRB was weakened under the high temperature condition. Most importantly, all these results are consistent with the single factor experiment.

Fig. 8 3D surface plots of 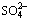 removal rate as function of pH and temperature (a), pH and ZPT concentration (b) and temperature and ZPT concentration (c)
removal rate as function of pH and temperature (a), pH and ZPT concentration (b) and temperature and ZPT concentration (c)
Response optimization technique contributed to identifying a production of a combination of input variables, which collectively optimized a single response or a set of responses [30]. It is useful to note that the goal of optimization is to find a good set of experimental conditions. The optimum conditions proposed by the model were initial pH 7.45, temperature 33.61 °C, and ZPT concentration 0.62 mg/L, at which maximum  removal rate of 91.62% was achieved. Similar results regarding to pH and Cr(VI) removal rate can be found in the work of AHMADI et al [27], where pH 7.5 was enough to reach the maximal Cr(VI) removal rate. In order to confirm the reliability and accuracy of the predicted value, the predicted conditions were validated by conducting an experiment twice for the reproducibility of the data [26]. As shown in Table 3, the
removal rate of 91.62% was achieved. Similar results regarding to pH and Cr(VI) removal rate can be found in the work of AHMADI et al [27], where pH 7.5 was enough to reach the maximal Cr(VI) removal rate. In order to confirm the reliability and accuracy of the predicted value, the predicted conditions were validated by conducting an experiment twice for the reproducibility of the data [26]. As shown in Table 3, the  removal rate of 91.37% (average of three replicates) was obtained by using optimized conditions, which was nearly 0.25% lower than the predicted value. The results were economically and technically feasible. It also confirmed that RSM was a powerful tool for optimizing the operational conditions of biodegradation experiment with great accuracy.
removal rate of 91.37% (average of three replicates) was obtained by using optimized conditions, which was nearly 0.25% lower than the predicted value. The results were economically and technically feasible. It also confirmed that RSM was a powerful tool for optimizing the operational conditions of biodegradation experiment with great accuracy.
Table 3 Process parameters for maximum 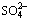 removal rate
removal rate

4 Conclusions
1) Sulfate reduction by SRB and consequent precipitation showed the potential to be cost-effective for simultaneous removal of high concentrations of Zn2+ and  from wastewater of ZPT production. High removal rates for both Zn2+ and
from wastewater of ZPT production. High removal rates for both Zn2+ and  in ZPT production wastewater reached under conditions of 30-35 °C, pH 7.0-8.0 and inoculum concentration of 10%-25%.
in ZPT production wastewater reached under conditions of 30-35 °C, pH 7.0-8.0 and inoculum concentration of 10%-25%.
2) ZPT inhibited the growth of SRB, resulting in the decrease of removal rates of Zn2+ and  . The inhibition by ZPT was enhanced with an increased ZPT concentration. In addition, the presence of Fe0 could not only enhance Zn2+ and
. The inhibition by ZPT was enhanced with an increased ZPT concentration. In addition, the presence of Fe0 could not only enhance Zn2+ and  removal rates, but also increase the resistance of SRB to ZPT toxicity.
removal rates, but also increase the resistance of SRB to ZPT toxicity.
3) The effects of variables using RSM, optimal operating conditions were found to be: pH 7.45, 33.61 °C, and ZPT concentration of 0.62 mg/L. Under these conditions, the removal rate of  was found to be 91.62%, which was consistent with the overlay plot results. Therefore, RSM could be effectively adopted to optimize the operating multifactor in complex biodegradation process.
was found to be 91.62%, which was consistent with the overlay plot results. Therefore, RSM could be effectively adopted to optimize the operating multifactor in complex biodegradation process.
References
[1] CARBAJO J B,  J A, PETRE A L, ROSAL R,
J A, PETRE A L, ROSAL R,  P,
P,  E. Personal care product preservatives: Risk assessment and mixture toxicities with an industrial wastewater [J]. Water Research, 2015, 72: 174-185.
E. Personal care product preservatives: Risk assessment and mixture toxicities with an industrial wastewater [J]. Water Research, 2015, 72: 174-185.
[2] JIAO Pan-pan, YANG Chun-ping, YANG Lei, DENG Zi-xi, SHAO Jing-jing, ZENG Guang-ming, YAN Zhou. Recovery of gallic acid from wastewater by extraction with tributyl phosphate/4-methyl-2- pentanone/n-hexane, tributyl phosphate/n-octanol/n-hexane and n-hexanol [J]. RSC Advances, 2016, 6: 93626-93639.
[3] SHAO Jing-jing, CHENG Yan, YANG Chun-ping, ZENG Guang-ming, LIU Wen-can, JIAO Pan-pan, HE Hui-jun. Efficient removal of naphthalene-2-ol from aqueous solutions by solvent extraction [J]. Journal of Environmental Sciences, 2016, 47(9): 120-129.
[4] LI Zhong-wu, HUANG Bin, HUANG Jin-quan, CHEN Gui-qiu, XIONG Wei-ping, NIE Xiao-dong, MA Wen-ming, ZENG Guang-ming. Influence of different phosphates on adsorption and leaching of Cu and Zn in red soil [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(2): 536-543.
[5] YANG Chun-ping, WANG Jia-qiang, LEI Min, XIE Geng-xin, ZENG Guang-ming, LUO Sheng-lian. Biosorption of zinc(II) from aqueous solution by dried activated sludge [J]. Journal of Environmental Sciences, 2010, 22(5): 675-680.
[6] FAN Ting, LIU Yun-guo, FENG Bao-ying, ZENG Guang-ming, YANG Chun-ping, ZHOU Ming, ZHOU Hai-zhou, TAN Zhen-feng, WANG Xin. Biosorption of cadmium(II), zinc(II) and lead(II) by Penicillium simplicissimum: Isotherms, kinetics and thermodynamics [J]. Journal of Hazardous Materials, 2008, 160(2-3): 655-661.
[7] AZABOU S, MECHICHI T, SAYADI S. Zinc precipitation by heavy-metal tolerant sulfate-reducing bacteria enriched on phosphogypsum as a sulfate source [J]. Minerals Engineering, 2007, 20: 173-178.
[8] ZHOU Qin, CHEN Yong-zhe, YANG Ming, LI Wen-kai, DENG Le. Enhanced bioremediation of heavy metal from effluent by sulfate-reducing bacteria with copper-iron bimetallic particles support [J]. Bioresource Technology, 2013, 136: 413-417.
[9] HE Hui-jun, CHEN Yu-juan, LI Xiang, CHENG Yan, YANG Chun-ping, ZENG Guang-ming. Influence of salinity on microorganisms in activated sludge processes: A review [J]. International Biodeterioration & Biodegradation, 2016, 119: 520-527.
[10] YAN Zhou, HE Hui-jun, YANG Chun-ping, ZENG Guang-ming, LUO Le, JIAO Pan-pan, WEN Shan, LI Hui-ru. Biodegradation of 3, 5-dimethyl-2, 4-dichlorophenol in saline wastewater by newly isolated Penicillium sp. yz11-22N2 [J]. Journal of Environmental Sciences, 2017, 57(7): 211-220.
[11] SOMASUNDARAM V, PHILIP L, BHALLAMUDI S M. Experimental and mathematical modeling studies on Cr(VI) reduction by CRB, SRB and IRB, individually and in combination [J]. Journal of Hazardous Materials, 2009, 172: 606-617.
[12] SINGH O V. Bio-nanoparticles: Biosynthesis and Sustainable Biotechnological Implications [M]. New Jersey: Wiley-Blackwell, 2015.
[13] POSTGATE J R. The sulphate-reducing bacteria [M]. Cambridge: Cambridge University Press, 1979.
[14] KUANG Fei, WANG Jia, YAN Li, ZHANG Dun. Effects of sulfate-reducing bacteria on the corrosion behavior of carbon steel [J]. Electrochimica Acta, 2007, 52: 6084-6088.
[15] BHOLA S M, ALABBAS F M, BHOLA R, SPEAR J R, MISHRA B, OLSON D L, KAKPOVBIA A E. Neem extract as an inhibitor for biocorrosion influenced by sulfate reducing bacteria: A preliminary investigation [J]. Engineering Failure Analysis, 2014, 36: 92-103.
[16] ABHILASH, PANDEY B D. Synthesis of zinc-based nanomaterials: A biological perspective [J]. IET Nanobiotechnology, 2012, 6(4): 144-148.
[17] ZHAO Mei-hua, ZHANG Chao-sheng, ZENG Guang-ming, HUANG Dan-lian, CHENG Min. Toxicity and bioaccumulation of heavy metals in Phanerochaete chrysosporium [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(5): 1410-1418.
[18] MARTINS M, FALEIRO M L, BARROS R J, VER SSIMO A R, BARREIROS M A, COSTA M C. Characterization and activity studies of highly heavy metal resistant sulphate-reducing bacteria to be used in acid mine drainage decontamination [J]. Journal of Hazardous Materials, 2009, 166: 706-713.
[19] UTGIKAR V P, HARMON S M, NAVENDU C, TABAK H H, RAKESH G, HAINES J R. Inhibition of sulfate-reducing bacteria by metal sulfide formation in bioremediation of acid mine drainage [J]. Environmental Toxicology, 2002, 17: 40-48.
[20] ZHANG Jing-xin, ZHANG Yao-bin, CHANG Jing-hui, QUAN Xie, LI Qi. Biological sulfate reduction in the acidogenic phase of anaerobic digestion under dissimilatory Fe (III)-reducing conditions [J]. Water Research, 2013, 47: 2033-2040.
[21] HUGHES M N, POOLE R K. Metal speciation and microbial growth-The hard (and soft) facts [J]. Microbiology, 1991, 137: 725-734.
[22] KUSHKEVYCH I V. Kinetic properties of pyruvate ferredoxin oxidoreductase of intestinal sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9 [J]. Polish Journal of Microbiology, 2015, 64: 107-114.
[23] PRUDEN A, MESSNER N, PEREYRA L, HANSON R E, HIIBEL S R, REARDON K F. The effect of inoculum on the performance of sulfate-reducing columns treating heavy metal contaminated water [J]. Water Research, 2007, 41: 904-914.
[24] YUE Zheng-bo, LI Qing, LI Chuan-chuan, CHEN Tian-hu, WANG Jin. Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria [J]. Bioresource Technology, 2015, 194: 399-402.
[25] BAI He, KANG Yong, QUAN Hon-gen, HAN Yang, FENG Ying. Treatment of copper wastewater by sulfate reducing bacteria in the presence of zero valent iron [J]. International Journal of Mineral Processing, 2012, 112-113: 71-76.
[26] QIU Lu, CHEN Yan, YANG Chun-ping, ZENG Guang-ming, LONG Zhi-yong, WEI Sai-nan, ZHAO Kun, LUO Le. Oxidative desulfurization of dibenzothiophene using a catalyst of molybdenum supported on modified medicinal stone [J]. RSC Advances, 2016, 6: 17036-17045.
[27] AHMADI R, REZAEE A, ANVARI M, HOSSINI H, RASTEGAR S O. Optimization of Cr(VI) removal by sulfate-reducing bacteria using response surface methodology [J]. Desalination & Water Treatment, 2015, 57(24): 11096-11102.
[28] PIKUTA E, LYSENKO A, SUZINA N, OSIPOV G, KUZNETSOV B, TOUROVA T, AKIMENKO V, LAURINAVICHIUS K. Desulfotomaculum alkaliphilum sp. nov.. A new alkaliphilic, moderately thermophilic, sulfate-reducing bacterium [J]. International Journal of Systematic & Evolutionary Microbiology, 2000, 50: 25-33.
[29] TECLU D, TIVCHEV G, LAING M, WALLIS M. Bioremoval of arsenic species from contaminated waters by sulphate-reducing bacteria [J]. Water Research, 2008, 42: 4885-4893.
[30] ZHAO Kun, CHENG Yan, LIU Hong-yu, YANG Chun-ping, QIU Lu, ZENG Guang-ming, HE Hui-jun. Extractive desulfurization of dibenzothiophene by a mixed extractant of N, N-dimethylacetamide, N, N-dimethylformamide and tetramethylene sulfone: Optimization by Box–Behnken design [J]. RSC Advances, 2015, 5: 66013-66023.
生物法处理吡啶硫酮锌生产废水中高浓度锌离子和硫酸根离子
彭志雄1,2,何慧军1,2,杨春平1,2,3,曾光明1,2,文 珊1,2,严 洲1,2,向海弘1,2,程 燕1,2,Sheldon TARRE4, Michal GREEN4
1. 湖南大学 环境科学与工程学院,长沙 410082;
2. 湖南大学 环境生物与控制教育部重点实验室,长沙 410082;
3. 浙江工商大学 环境科学与工程学院 浙江省固体废物处理与资源化重点实验室,杭州 310018;
4. Faculty of Civil and Environmental Engineering, Israel Institute of Technology, Haifa 32000, Israel
摘 要:采用富集和驯化后的硫酸盐还原菌处理吡啶硫酮锌生产废水,并考察不同实验参数对硫酸盐降解和锌沉淀的影响。单因素实验结果表明:当吡啶硫酮锌浓度为3.0~5.0 mg/L时,锌离子和硫酸根离子的去除率呈明显下降趋势。当温度为30~35 °C、pH为 7~8、接种量为10%~25%时,锌离子和硫酸根离子都能得到有效去除。零价铁不仅能提高硫酸盐还原菌对硫酸根离子和锌离子的去除效果,还能加强硫酸盐还原菌对锌离子和吡啶硫酮锌毒性的抗性。此外,采用Box-Behnken法研究主要实验参数对硫酸盐去除率的影响。得到最佳实验条件如下: pH 7.45、温度 33.61 °C、吡啶硫酮锌浓度 0.62 mg/L。在最佳实验条件下硫酸根离子的去除率能达到最大值91.62%。
关键词:生物处理;硫酸盐还原;硫酸盐还原菌;废水;吡啶硫酸锌;锌
(Edited by Wei-ping CHEN)
Foundation item: Project (2015DFG92750) supported by the International S&T Cooperation Program of China; Projects (51278464, 51478172) supported by the National Natural Science Foundation of China; Project (2014GK1012) supported by the Department of Science and Technology of Hunan Province, China
Corresponding author: Chun-ping YANG; Tel: +86-731-88823987; E-mail: yangc@hnu.edu.cn
DOI: 10.1016/S1003-6326(17)60275-9
Abstract: An enriched and domesticated bacteria consortium of sulfate-reducing bacteria (SRB) was used to treat wastewater from zinc pyrithione (ZPT) production, and the effects of different reaction parameters on sulfate reduction and zinc precipitation were evaluated. The single-factor experimental results showed that the removal rates of Zn2+ and 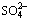 decreased with an increased ZPT concentration ranging from 3.0 to 5.0 mg/L. Zn2+ and
decreased with an increased ZPT concentration ranging from 3.0 to 5.0 mg/L. Zn2+ and 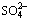 in wastewater were effectively removed under the conditions of 30-35 °C, pH 7-8 and an inoculum concentration of 10%-25%. The presence of Fe0 in the SRB system enhanced Zn2+ and
in wastewater were effectively removed under the conditions of 30-35 °C, pH 7-8 and an inoculum concentration of 10%-25%. The presence of Fe0 in the SRB system enhanced Zn2+ and 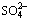 removal and may increase the resistance of SRB to the toxicity of Zn2+ and ZPT in wastewater. A Box-Behnken design was used to evaluate the influence of the main operating parameters on the removal rate of
removal and may increase the resistance of SRB to the toxicity of Zn2+ and ZPT in wastewater. A Box-Behnken design was used to evaluate the influence of the main operating parameters on the removal rate of 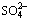 . The optimum parameter values were found to be pH 7.45, 33.61 °C and ZPT concentration of 0.62 mg/L, and the removal rate of
. The optimum parameter values were found to be pH 7.45, 33.61 °C and ZPT concentration of 0.62 mg/L, and the removal rate of 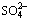 reached a maximum of 91.62% under these optimum conditions.
reached a maximum of 91.62% under these optimum conditions.


