文章编号:1004-0609(2012)05-1468-07
水对AlCl3-BMIC离子液体电沉积铝的影响
裴启飞,华一新,李 艳,崔 焱,刘成虎
(昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:
采用循环伏安和线性扫描伏安法研究水对摩尔比为2:1的AlCl3-BMIC离子液体电化学性质的影响。结果表明:随着含水量的增加,铝的还原峰电流密度减小,阴极和阳极极化过电位增加,槽电压不断升高。当离子液体含水量低于1.39 mol/L时,电流效率稳定在94%~95%;继续增加含水量到1.67 mol/L时,电流效率从94%下降到64.7%。当离子液体含水量低于1.39 mol/L时,沉积层变得更致密;当含水量增加到1.67 mol/L时,沉积层变得疏松。离子液体含水量为1.39 mol/L时,铝的纯度超过99.9%。
关键词:
中图分类号:TF111 文献标志码:A
Effect of water on electrodeposition of aluminum from AlCl3-BMIC ionic liquid
PEI Qi-fei, HUA Yi-xin, LI Yan, CUI Yan, LIU Cheng-hu
(Faculty of Metallurgical and Energy Engineering, Kunming University of science and Technology,
Kunming 650093, China)
Abstract: The effect of water on electrochemical characteristics of AlCl3-BMIC ionic liquid with molar ratio of 2:1 was investigated by cyclic voltammetry and linear sweep voltammetry. The results show that the cathodic peak current density decreases, the overpotential values of cathode and anode increase and the cell voltage increases with water concentration increasing in the ionic liquid. Furthermore, the cell voltage, current efficiency and surface morphology of aluminum electrodeposits are affected by amount of water in ionic liquid. The current efficiency is various in the range from 94% to 95% when the water concentration is below 1.39 mol/L, while it decreases sharply from 94% to 64.7% when the water concentration is increased to 1.67 mol/L. The deposit layer becomes more compact when the water concentration of ionic liquid is below 1.39 mol/L, but it becomes loosen when the water concentration is increased to 1.67 mol/L. The purity of aluminum deposit is over 99.9% when the water concentration is 1.39 mol/L.
Key words: water; ionic liquid; electrodeposition; aluminum
离子液体是由有机阳离子和无机阴离子组成在低温(一般低于100 ℃)下呈液态的熔盐。与常规溶剂相比,离子液体具有电化学窗口宽、电导率和离子迁移率高、可室温操作、几乎不挥发、毒性小等优点[1-2]。这些离子液体的优点为铝电解精炼提供了一种新的途径。
目前,研究者已从AlCl3-TMPAC、AlCl3-EMIC、AlCl3-BPC等离子液体中电沉积出铝[3-14],并通过添加剂(如苯、甲苯等)改善沉积层质量。KAMAVARAM 等[15]在100 ℃下用AlCl3-BMIC离子液体作为电解质电解精炼含铝79.77%的铝合金A360,可在阴极上得到纯度为99.89%的电解铝,电流效率可达98%,能耗仅为3.0 kW·h/kg-Al,比传统三层液电解精炼铝的能耗15~18 kW·h/kg-Al降低了80%~83%,操作温度降低660~710 ℃。李艳等[16]采用摩尔比为2:1的AlCl3-BMIC离子液体电解精炼纯度为92.12%的铝合金,在阴极铜基体上获得纯度超过99.9%的金属铝,且直流电耗最低仅为0.611 kW·h/kg-Al。
目前,用于电沉积铝的离子液体以AlCl3型离子液体为主。但是,此类离子液体在空气中易吸水,并且会与之发生强烈的化学反应。SAHAMI和OSTERYOUHG[17]以铂为工作电极采用伏安法研究了水对碱性AlCl3-BPC离子液体中电化学行为的影响,研究发现,加水后离子液体的循环伏安曲线与加水前相比发生了明显的变化;还发现离子液体与水反应生成了HCl且溶于离子液体中。ABBOTT等[18]研究发现,在1 kg 的AlCl3-BTMAC离子液体中存在0.5 mol水时,水对其电化学性质仅有很小的影响。本文作者将水滴加到摩尔比为2:1的AlCl3-BMIC离子液体中,观测到离子液体与水发生强烈的化学反应,生成白色物质并溶于离子液体,同时放出HCl气体。当 AlCl3-BMIC离子液体中有水存在时,可能会对其电化学性质以及铝的电沉积产生影响。因此,研究水对离子液体电解精炼铝的影响,深入了解水在电解精炼过程中的行为,对推动离子液体电解精炼铝新技术的发展具有重要的作用。
1 实验
1.1 试剂
实验所用的氯代正丁烷、N-甲基咪唑、无水三氯化铝、甲苯、分子筛等均为市售AR试剂。氯代正丁烷、乙酸乙酯和N-甲基咪唑在使用前分别用分子筛除水,然后进行蒸馏提纯;甲苯先用分子筛除水,再用浓硫酸萃取除去甲基噻吩,然后蒸馏提纯。其它试剂未做进一步处理。
1.2 实验方法
经提纯处理过的氯代正丁烷和N-甲基咪唑,按摩尔比1.2:1的量加入三颈瓶中,甲苯作为溶剂,在80 ℃,氩气保护气氛下,搅拌回流48 h。反应结束后,产物经分层除去部分溶剂和未反应物,用乙酸乙酯反复洗涤,减压蒸馏残留的乙酸乙酯,80 ℃下真空干燥12 h以上,得到淡黄色黏稠性的氯化1-丁基-3-甲基咪唑(BMIC)液体。
在真空手套箱中按摩尔比为2:1的量分别称取AlCl3和BMIC,将BMIC置于三口瓶中,在氩气保护气氛下,分批加入AlCl3并不断搅拌,三口瓶用冰水浴冷却。反应完毕,得到淡黄色的AlCl3-BMIC离子液体,将其分装到玻璃杯中密封备用。
在离子液体中加入超纯水,待水与离子液体反应生成的白色物质完全溶解之后再进行电化学测试。电化学测试采用PCI4/300型电化学工作站,循环伏安测试以玻碳(GC)电极(d=4 mm)作为工作电极,铂(Pt)丝作为辅助电极,高纯铝丝(99.99%)插入摩尔比为2:1的AlCl3-BMIC离子液体中作参比电极,装有参比离子液体的玻璃管底端采用多孔陶瓷密封。实验前,工作电极经晶相砂纸打磨平整,然后用0.5 μm的Al2O3粉抛光至镜面,再依次放入丙酮、稀硝酸中用超声波清洗,超纯水冲洗干净。Pt电极用抛光粉打磨光亮,然后用丙酮、超纯水超声清洗。参比铝丝依次用去污剂、丙酮和超纯水清洗。
在氩气保护气氛下分别将不同量的水加入到离子液体中,然后在80 ℃下用磁力搅拌足够长的时间使水与离子液体反应生成的物质完全溶解在离子液体中,并在离子液体中放置纯铝片(99.95%)以去除溶于离子液体中的HCl,取出纯铝片制得不同含水量的离子液体电解质。
恒电流电解电沉积采用CHI760D型电化学工作站,电解时用磁力搅拌并保持恒定搅拌速度,在80 ℃下沉积4 h,电流密度为10 mA/cm2。电解时将电化学工作站的参比电极与辅助电极一起连接到阳极,所测电势即为槽电压,取平均值为整个电解过程槽电压。采用双阳极电解,纯铝片(99.95%)作阳极,浸入离子液体面积为1.0 cm×1.0 cm的纯铜片(99.95%)作阴极,电极间距1 cm。电沉积前,阴极铜片先用砂纸打磨至光亮无划痕,然后清洗干净,冷风吹干,称量备用。实验完毕,取出阴极放入乙腈溶液溶去沉积层表面的离子液体,蒸馏水清洗干净,干燥称量。采用XL30ESEM-TMP型扫描电子显微镜(SEM)及附带的能谱分析仪(EDS)分析铝沉积层的形貌和纯度。
电流效率(η)=(实际电沉积铝的质量/阴极所通过电量下理论沉积铝的质量)×100%。
2 结果与讨论
2.1 电化学测试
采用循环伏安(CV)和线性扫描伏安(LSV)测定水对摩尔比为2:1的AlCl3-BMIC离子液体电化学性质的影响。图1所示为不同含水量离子液体在GC电极上的CV曲线,电位扫描从1 V开始,向负方向移动,达到-1 V之后回扫到1 V,扫速为50 mV/s。由图1中纯AlCl3-BMIC离子液体的CV曲线可以看出,当电位从1 V移动到B点附近时,铝开始在GC电极上沉积,随着电位的不断负移,还原峰C1出现,电位回扫到B点附近时,沉积在GC电极上的铝开始被氧化,随着电位的正移,氧化峰A1出现。离子液体中铝的还原和氧化反应方程如下:
1) 还原反应
![]() →
→![]() (1)
(1)
2) 氧化反应
![]() →
→![]() (2)
(2)
当离子液体含水时,随着含水量的增加,还原峰(C1~C5)和氧化峰(A1~A4)的峰值电流密度逐渐减小。
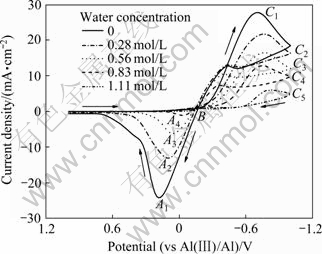
图1 含水量对AlCl3-BMIC离子液体循环伏安曲线的影响
Fig. 1 Effect of water concentration on cyclic voltammo- grams of AlCl3-BMIC ionic liquid
由式(1)可知,离子液体中还原出铝的配阴离子是![]() ,还原电流密度jc可用下式表示[19]:
,还原电流密度jc可用下式表示[19]:
![]() (3)
(3)
式中:n为反应所转移的电子数,0.75;R为气体常数,8.314 J/(K·mol);T为热力学温度,K;![]() 为
为![]() 的扩散系数,cm2/s;
的扩散系数,cm2/s;![]() 为
为![]() 物质的量浓度,mol/cm3;v为扫描速率,V/s;Ψ(E)为准可逆电流函数;由式(3)可知,还原电流密度与
物质的量浓度,mol/cm3;v为扫描速率,V/s;Ψ(E)为准可逆电流函数;由式(3)可知,还原电流密度与![]() 的扩散系数、浓度及Ψ(E)的值有关,而扩散系数又与离子液体的黏度有关[20]:
的扩散系数、浓度及Ψ(E)的值有关,而扩散系数又与离子液体的黏度有关[20]:
![]() (4)
(4)
式中:kB为玻尔兹曼常量,1.38×10-23 J/K;![]() 为
为![]() 离子半径,m。
离子半径,m。
在摩尔比为2:1的AlCl3-BMIC离子液体中,含铝的配阴离子有![]() 、
、![]() 和
和![]() ,其中
,其中![]() 占主要部分[21]。当离子液体中有水存在时,水会与离子液体中的
占主要部分[21]。当离子液体中有水存在时,水会与离子液体中的![]() 、
、![]() 和
和![]() 反应生成
反应生成![]() 、
、![]() 、
、![]() 和
和![]() 等多种含铝配阴离子[22-23]。水的存在不仅消耗了
等多种含铝配阴离子[22-23]。水的存在不仅消耗了![]() 、
、![]() 和
和![]() 的数量,使离子浓度降低,而且反应生成的一些离子较大的含铝配阴离子会增加离子液体的黏度,使
的数量,使离子浓度降低,而且反应生成的一些离子较大的含铝配阴离子会增加离子液体的黏度,使![]() 和
和![]() 等离子扩散受阻,此外,离子液体含水也可能会对Ψ(E)的值产生影响。由式(3)可知,液体中
等离子扩散受阻,此外,离子液体含水也可能会对Ψ(E)的值产生影响。由式(3)可知,液体中![]() 浓度降低和扩散系数减小,都会使峰值电流密度降低。还原峰值电流密度降低,还原电位下在玻碳电极上沉积铝的量减少,对应的氧化峰值电流密度也降低。
浓度降低和扩散系数减小,都会使峰值电流密度降低。还原峰值电流密度降低,还原电位下在玻碳电极上沉积铝的量减少,对应的氧化峰值电流密度也降低。
不同含水量下阴极与阳极LSV曲线如图2所示,电位扫描分别为-0.1~-0.4和0~0.45 V,扫描速度为5 mV/s。由图2可以看出,含水量对铝的析出电位和阳极起始氧化电位影响不大,随着离子液体含水量的增加,阴极和阳极极化过电位增加。与铝在GC电极上的还原原理相同,随着水量的增加,铜电极表面可以还原出铝的![]() 浓度不断降低,导致电流密度下降,即电流密度一致时,需要更负的电位。根据式(2)可知,离子液体中
浓度不断降低,导致电流密度下降,即电流密度一致时,需要更负的电位。根据式(2)可知,离子液体中![]() 浓度的降低和扩散系数的减小导致阳极极化过电位增高。
浓度的降低和扩散系数的减小导致阳极极化过电位增高。
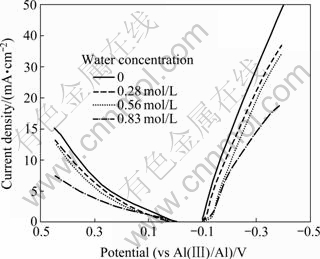
图2 含水量对阴极和阳极极化的影响
Fig. 2 Effect of water concentration on polarisation behavior of cathode and anode
2.2 水对铝电沉积槽电压的影响
图3所示为含水量与槽电压的关系。由图3可以看出,槽电压随离子液体含水量的增加而升高。电解槽接触良好,外阻可以忽略,槽电压Ec可用式(5)来表示[24]:
![]() (5)
(5)
式中:ηA和ηC分别为阳极和阴极过电位,V;I为通过电解液的电流,A;R为电解液的内阻,Ω。由式(5)可知,槽电压与阴极和阳极极化过电位及电解液的内阻有关。
由图2中的阴极和阳极极化曲线可知,阴极和阳极极化过电位都随着含水量的增加而增大。此外,离子液体与水反应的生成物也会降低离子液体的电导率,增大离子液体的内阻。受两者的共同影响,槽电压随着含水量的增加而升高。
根据铝电解精炼的直流电单耗(W)与槽电压的关系有[25]
![]() (6)
(6)
式中:Eave为平均槽电压,V;ηeff为电流效率,%;Q为铝的电量系数,2 980 A·h/kg。由式(6)可知,在电流效率一定的情况下,随着槽电压升高,直流电单耗增加,这对铝的电解精炼不利。
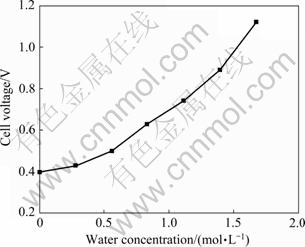
图3 含水量对槽电压的影响
Fig. 3 Effect of water concentration on cell voltage
2.3 水对铝电沉积电流效率的影响
含水量对铝电沉积电流效率的影响如图6所示。由图6可以看出:纯离子液体中铝在铜基体上电沉积的电流效率为94.4%;当含水量不超过1.39 mol/L时,铝电沉积的电流效率稳定在94%~95%之间,继续增加含水量到1.67 mol/L时,铝沉积的电流效率突然下降到64.7%。
LAI等[26]研究发现,离子液体中的有机阳离子和杂质会腐蚀铝沉积层。在纯离子液体中铝电沉积的电流效率只有94.4%,可能是阴极沉积出的铝被腐蚀而返溶到离子液体中及溶液电阻消耗了部分电量的缘故。离子液体含水量不超过1.39 mol/L时,离子液体中的![]() 浓度仍能够满足铝的电沉积,水对电流效率影响不大。当含水量增大到1.67 mol/L时,阴极表面的
浓度仍能够满足铝的电沉积,水对电流效率影响不大。当含水量增大到1.67 mol/L时,阴极表面的![]() 浓度不能满足铝电沉积需要的量,使电流效率急剧降低。
浓度不能满足铝电沉积需要的量,使电流效率急剧降低。
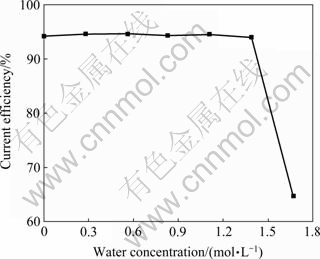
图4 含水量对电流效率的影响
Fig. 4 Effect of water concentration on current efficiency
2.4 沉积层形貌分析
在电流密度为10 mA/cm2的条件下,沉积4 h之后,根据阴极铝致密层的增质量算得铝沉积层平均厚度约为40 μm。图5(a)所示为纯离子液体中铝沉积层形貌。由图5(a)可以看出,沉积层表面由松散的块状、柱状和片状颗粒组成,颗粒之间的间距较大,表面不致密平整。图5(b)所示为离子液体中加入0.28 mol/L水之后沉积层的表面形貌,由图5(b)可以看出,沉积层的铝颗粒变得细化,表面致密均匀,沉积层呈片状结构生长。随着含水量的增加,沉积层的铝颗粒变得更细化,表面也更加致密均匀,如图5(c)和(d)所示。由图2中的阴极极化曲线可以看出,随着含水量的增加,阴极极化过电位增高,过电位增高,晶粒的生长受阻,晶核的形成速度加快,沉积层结晶细致。而当含水量达到1.67 mol/L时,阴极表面缺少放电的![]() ,金属离子一般在棱角和凸出部位放电,出现大块的颗粒,如图5(e)所示。
,金属离子一般在棱角和凸出部位放电,出现大块的颗粒,如图5(e)所示。
图6所示为离子液体含水量为1.39 mol/L时铝沉积层的EDS谱。由图6可以看出,铝的峰很强,离子液体即使含有少量的水仍然可以沉积出纯度超过99.9%的铝。

图5 含水量对沉积层形貌的影响
Fig. 5 Effect of water concentration on surface morphologies of aluminum electrodeposits: (a) Blank; (b) 0.28 mol/L; (c) 0.83 mol/L; (d) 1.39 mol/L; (e) 1.67 mol/L

图6 铝沉积物的EDS谱
Fig. 6 EDS spectrum of aluminum electrodeposit
3 结论
1) 在摩尔比为2:1的AlCl3-BMIC离子液体中存在少量的水仍然可以沉积铝。
2) 随着含水量的增加,氧化还原峰值电流密度减小,阴极和阳极极化过电位增高。
3) 铝电沉积槽电压随着含水量的增加不断地升高。
4) 当含水量不超过1.39 mol/L时,水对电流效率影响不大,电流效率在94%~95%之间;继续增加含水量到1.67 mol/L时,铝沉积的电流效率突然下降到64.7%。
5) 离子液体中含水量不超过1.39 mol/L时,少量的水可以细化沉积层铝颗粒,改善铝的沉积层质量,使沉积层变的致密均匀;当含水量较高达到1.67 mol/L时,沉积层变得疏松、易脱落。
REFERENCES
[1] 李汝雄. 绿色溶剂-离子液体的合成及应用[M]. 北京: 化学工业出版社, 2004: 10-27.
LI Ru-xiong. Green solvent-synthesis and application of ionic liquid[M]. Beijing: Chemical Industry Press, 2004: 10-27.
[2] 邓友全. 室温离子液体-新型的精细化学品[J]. 精细化工原料及中间体, 2007(10): 3-4.
DENG You-quan. Room temperature ionic liquid-new fine chemicals[J]. Fine Chemical Industrial Raw Materials and Intermediates, 2007(10): 3-4.
[3] ZHAO Yu-guang, VANDERNOOT T J. Electrodeposion of aluminum from room temperature AlCl3-TMPAC molten salt[J]. Electrochimica Acta, 1997, 42(11): 1639-1643.
[4] 高丽霞, 王丽娜, 齐 涛, 李玉平, 初景龙, 曲景奎. 离子液体AlCl3/Et3NHCl中电沉积制备金属铝[J]. 物理化学学报, 2008, 24(6): 939-944.
GAO Li-xia, WANG Li-na, QI Tao, LI Yu-ping, CHU Jing-long, QU Jing-kui. Electrodeposition of aluminum from AlCl3/Et3NHCl ionic liquids[J]. Acta Physico-Chimica Sinica, 2008, 24(6): 939-944.
[5] 张丽鹏, 于先进, 洪淑翠, 董云会, 李德刚, 张 欣. 铝基离子液体(BMIM)Br-AlCl3的制备与电沉积应用[J]. 中国有色金属学报, 2008, 18(S1): s274-s278.
ZHANG Li-peng, YU Xian-jin, HONG Shu-cui, DONG Yun-hui, LI De-gang, ZHANG Xin. Preparation and electrodeposition of Al-based ionic liquid (BMIM)Br-AlCl3[J]. The Chinese Journal of Nonferrous Metals, 2008(S1): s274-s278.
[6] KOURA N, NAGASE H, SATO A,KUMAKURA S, TAKEUCHI K, UI K, TSUDA T, LOONG K. Electroless plating of aluminum from a room-temperature ionic liquid electrolyte[J]. Journal of the Electrochemical Society, 2008, 155(2): D155-D157.
[7] ZEIN EI ABEDIN S, MOUSTAFA E M, HEMPELMANN R, NATTER H, ENDRES F. Additive free electrodeposition of nanocrystalline aluminium in a water and air stable ionic liquid[J]. Electrochemistry Communications, 2005, 7(11): 1111-1116.
[8] ZEIN EI ABEDIN S, MOUSTAFA E M, HEMPELMANN R, NATTER H, ENDRES F. Electrodeposition of nano- and microcrystalline aluminium in three different air and water stable ionic liquids[J]. Chem Phys Chem, 2006, 7(7): 1535-1543.
[9] JIANG T, CHOLLIER BRYM M J, DUB? G, LASIA A, BRISARD G M. Electrodeposition of aluminium from ionic liquids: Part I-electrodeposition and surface morphology of aluminium from aluminium chloride (AlCl3)–1-ethyl-3- methylimidazolium chloride ([EMIm]Cl) ionic liquids[J]. Surface and Coatings Technology, 2006, 201(1/2): 1-9.
[10] KAMAVARAM V, MANTHA D, REDDY R G. Recycling of aluminum metal matrix composite using ionic liquids: Effect of process variables on current efficiency and deposit characteristics[J]. Electrochimica Acta, 2005, 50(16/17): 3286-3295.
[11] YANG Chao-cheng. Electrodeposition of aluminum in molten AlCl3-n-butylpyridinium chloride electrolyte[J]. Materials Chemistry and Physics, 1994, 37(4): 355-361.
[12] CAPORALI S, FOSSATI A, LAVACCHI A, PERISSI I, TOLSTOGOUZOV A, BARDI U. Aluminium electroplated from ionic liquids as protective coating against steel corrosion[J]. Corrosion Science, 2008, 50(2): 534-539.
[13] LIU Q X, ZEIN EL ABEDIN S, ENDRES F. Electrodeposition of nanocrystalline aluminum: Breakdown of imidazolium cations modifies the crystal size[J]. The Journal of Physical Chemistry B, 2008, 155(5): D357-D362.
[14] MOUSTAFA E M, ZEIN EL ABEDIN S, SHKURANKOV A. Electrodeposition of Al in 1-Butyl-1-methylpyrrolidinium bis (trifluoromethylsulfonyl)amide and 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ionic liquids: In situ STM and EQCM studies[J]. The Journal of Physical Chemistry B, 2007, 111(18): 4693-4707.
[15] KAMAVARAM V, MANTHA D, REDDY R G. Electrorefining of aluminum alloy in ionic liquids at low temperatures[J]. Journal of Mining and Metallurgy, 2003, 39 (1/2)B: 43-58.
[16] 李 艳, 华一新, 张启波, 裴启飞, 崔 炎, 徐存英, 田国才. 氯化胆碱添加剂对[Bmim]Cl-AlCl3离子液体体系电解精炼铝的影响[J]. 过程工程学报, 2010, 10(5): 981-986.
LI Yan, HUA Yi-xin, ZHANG Qi-bo, PEI Qi-fei, CUI Yan, XU Cun-ying, TIAN Guo-cai. Effects of additive choline chloride on electrolysis of aluminum from [Bmim]Cl-AlCl3 ionic liquid system[J]. The Chinese Journal of Process Engineering, 2010, 10(5): 981-986.
[17] SAHAMI S, OSTERYOUNG R A. Voltammetric determination of water in an aluminum chloride-N-n-butylpyridinium chloride ionic liquid[J]. Analytical Chemistry, 1983, 55(12): 1970-1973.
[18] ABBOTT A P, EARDLEY C A, FARLEY N R S, PRATT A. Novel room temperature molten salts for aluminium electrodeposition[J]. Transactions of the Institute of Metal Finishing, 1999, 77(1): 26-28.
[19] 阿伦·J·巴德, 拉里·R·福克纳. 电化学原理: 方法与应用[M]. 邵元华,朱果逸,董献堆,张柏林, 译. 北京:化学工业出版社, 2005: 164.
BARD A J, FAULKNER L R. Electrochemical methods: Fundamentals and applications[M]. SHAO Yuan-hua, ZHU Guo-yi, DONG Xian-dui, ZHANG Bo-lin, transl. Beijing: Chemical Industry Press, 2005: 164.
[20] 高 颖, 邬 冰. 电化学基础[M]. 北京: 化学工业出版社, 2004: 11-14.
GAO Ying, WU Bing. Electrochemistry foundation[M]. Beijing: Chemical Industry Press, 2004: 11-14.
[21] MELTON T J, JOYCE J, MALOY J T,BOON J A, WILKES J S. Electrochemical studies of sodium chloride as a lewis buffer for room temperature chloroaluminate molten salts[J]. Journal of the Electrochemical Society, 1990, 137(12): 3865-3869.
[22] ABDUL-SADA A K, GREENWAY A M. A fast atom bombardment mass spectrometric study of room-temperature l-ethyl-3-methylimidazolium chloroaluminate(Ⅲ) ionic liquids: evidence for the existence of the decachlorotrialuminate(Ⅲ) anion[J]. Organic Mass Spectrometry, 1993, 28(7): 759-765.
[23] ZAWODZINSKI T A Jr, OSTERYOUNG R A. Oxide and hydroxide species formed on addition of water in ambient-temperature chloroaluminate melts: An 17O NMR study[J]. Inorganic Chemistry, 1990, 29(15): 2842-2841.
[24] 傅崇说. 有色冶金原理[M]. 北京: 冶金工业出版社, 2005: 285.
FU Chong-yue. Principle of non-ferrous metallurgy[M]. Beijing: Metallurgical Industry Press, 2005: 285.
[25] 邱竹贤. 铝电解[M]. 北京: 冶金工业出版社, 1995: 180.
QIU Zhu-xian. Aluminum electrolysis[M]. Beijing: Metallurgical Industry Press, 1995: 180.
[26] LAI P K, SKYLLAS-KAZACOS M. Electrodeposition of aluminum in aluminum chloride/1-methyl-3-ethylimidazolium chloride[J]. Journal of Electroanalytical Chemistry, 1988, 248(2): 431-440.
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(50864009,50904031);高等学校博士学科点专项科研基金资助项目(20070674001)
收稿日期:2010-01-10;修订日期:2011-05-19
通信作者:华一新,教授, 博士;电话:0871-5162008;E-mail: huayixin@gmail.com
摘 要:采用循环伏安和线性扫描伏安法研究水对摩尔比为2:1的AlCl3-BMIC离子液体电化学性质的影响。结果表明:随着含水量的增加,铝的还原峰电流密度减小,阴极和阳极极化过电位增加,槽电压不断升高。当离子液体含水量低于1.39 mol/L时,电流效率稳定在94%~95%;继续增加含水量到1.67 mol/L时,电流效率从94%下降到64.7%。当离子液体含水量低于1.39 mol/L时,沉积层变得更致密;当含水量增加到1.67 mol/L时,沉积层变得疏松。离子液体含水量为1.39 mol/L时,铝的纯度超过99.9%。


