
Valence electron structures and properties of Ni-based corrosion resistant alloy
YANG Rui-cheng(杨瑞成), SHU Jun(舒俊), CHEN Kui(陈奎), WANG Kai-xuan(王凯旋)
State Key Laboratory of Gansu Advanced Non-ferrous Metal Materials, Lanzhou University of Technology,
Lanzhou 730050, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The corrosion resistance and mechanical properties were tested and compared for the newly synthesized as-cast, as-solution Ni-Cr-Mo-Cu corrosion resistant alloys and 1Cr18Ni9Ti austenitic stainless steel. Their valence electron structural units were constructed, and the relative parameters were calculated by means of the Empirical Electron theory of Solids and Molecules (EET). The results show that, during alloy elements Cr, Mo and Cu entering Ni-matrix, the bonding strength nA and bonding energy EA of the strongest bond of the alloy are greatly increased, causing the stronger solid solution strengthening effects (about 30% increase in σb). Also, as reinforcement of the main bond network and the improvement of stability of the alloy system due to the solution of these alloying elements in γ-Ni, the ionization of metal atoms in corrosion solution and the flow of electrons from anode to cathode would all be impeded during electro-chemical corrosion processes, which leads to the excellent corrosion resistant ability of the present Ni-Cr-Mo-Cu alloy (about 2-3 orders of magnitude as high as 1Cr18Ni9Ti austenitic stainless steel) in several highly aggressive solutions.
Key words:
nickel-base corrosion-resistant alloy; valence electron structure; solid solution effect; corrosion resistance;
1 Introduction
The corrosion resistant materials, as a member of ecomaterials, play an important role in coordinating the relationship between materials and environment. Comparable with stainless steel, other corrosion resistant metals and non-metallic materials, nickel-base alloys can provide not only superior corrosion resistance to various types of chemicals, but also good mechanical properties and workability[1]. Researches and practices[1-3] have shown that Ni-base alloys are most suitable for applications in highly aggressive environment, sometimes being unique choice, such as containing-rich iron and chloric ions. Recently, Corrosion behaviors of NiCrFe 600 alloy and NiCrMo 625 alloy in high temperature, hydrogenated water and in acetic acid solution were reported[4-6], and researches on improved pitting corrosion behavior and electrochemical corrosion behavior of Ni-base alloy coatings[7-9] by electrodeposited nanocrystalline[7] and double glow plasma alloying technique[8] were emphasized.
Adding Cr, Mo, W and Cu etc to pure nickel, Ni-base alloys can be endowed with more excellent properties, which constructs a wide range of nickel-base corrosion resistant alloys. However, up to now, the nature and micro-mechanism of its corrosion resistant behavior have not been clear from the point of view of materials science.
Based on the tests of mechanical and corrosive properties for the present newly developed Ni-Cr-Mo-Cu alloy, its valence electron structures were calculated and analyzed by means of the Empirical Electron Theory of solid and molecules (EET)[10] in order to explore its solution effects and natures of outstanding corrosion properties, which has not been seen in recent published works and should have obvious scientific and engineering significance.
2 Synthesis and properties of tested Ni-Cr- Mo-Cu alloy
Based on new progress in corrosive resistant alloy, the main composition (mass fraction, %) ranges of the present designed alloys are Cr 20-25, Mo 14-20, Ni≥60 and some of copper (≤3) according to the APF (=4Cr/(2Mo+W)) factor as a relevant value. After melted in consumable electrode vacuum arc furnace (about 5 kg, see Fig.1), the solution treatment(2.5 h, see Fig.2) at 1 140-1 170 ℃ was done for homogenizing and then water-quenched for anstenitizing[11]. Its mechanical properties are shown in Table 1. The corrosive tests of the present Ni-Cr-Mo-Cu alloy were performed at 90 ℃ for 100 h in 50% HNO3, 30% HCl, 15% FeCl3 solutions and mixed acid (20 mL 36.4% HCl+20 mL 65%HNO3+50 mL H2O), and its results of as-cast and as-solution conditions are all shown in Table 2 (Taking austenitic stainless steel 1Cr18Ni9Ti for comparison).
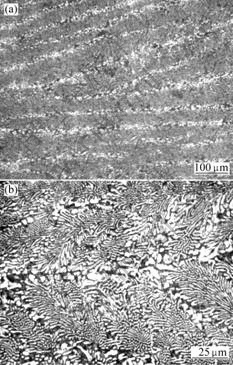
Fig.1 As-cast microstructures of Ni-Cr-Mo-Cu alloy melted at consumable electrode vacuum arc furnace
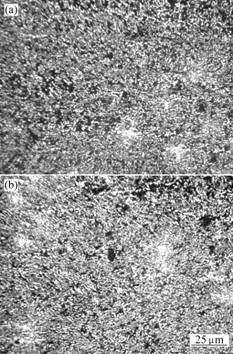
Fig.2 As-solution condition microstructures of Ni-Cr-Mo-Cu alloy: (a) 1 140 ℃+2.5 h; (b) 1 170 ℃+2.5 h
Table 1 Mechanical properties of as-cast and as-solution conditions of Ni-Cr-Mo-Cu alloy

Table 2 Comparisons of corrosion resistance between as-cast and as-solution conditions of Ni-Cr-Mo-Cu alloy and 1Cr18Ni9Ti austenitic stainless steel
3 Establishment of valence electron structural models of Ni-Cr-Mo-Cu alloy
According to EET[10], the main valence electron structural (VES) units of Ni-Cr-Mo-Cu alloy change from the single γ-Ni unit of pure nickel into γ-Ni, γ-(Ni-Cr), γ-(Ni-Mo), γ-(Ni-Cu), γ-(Ni-Cr-Mo), γ-(Ni-Cr-Cu), γ-(Ni-Mo-Cu), γ-(Ni-Cr-Mo-Cu) units, and obviously, the units containing copper have a few proportion. The calculating models of these structural units (γ-Ni, γ-(Ni-Me), γ-(Ni-Mex-Mey) and γ-(Ni-Mex-Mey-Mez)) are shown in Fig.3, in consideration of the symmetry of structural unit[10].
4 Calculation results and analysis
The main calculated results of VES parameters of the above structural units are shown in Table 3 by means of the bond length difference (BLD) method[10] of EET, in which nA, nB and EA, EB are the co-valence electron pairs and their bond energy per mole of the strongest bond and the second one, respectively, E is the total binding energy of all interatomic bond in unit (considering the contribution of lattice electrons and magnetic electrons), and ∑nα is the total co-valence electron pairs in structural unit. Table 4 lists the results of 1Cr18Ni9Ti as the comparison object.
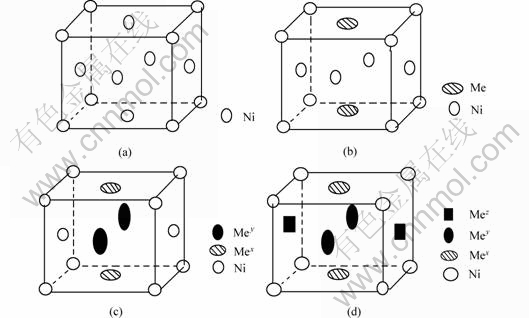
Fig.3 Calculation models of structural units of Ni-base corrosion resistant alloy: (a)γ-Ni unit; (b)γ-(Ni-Me) unit; (c) γ-(Ni-Mex-Mey ) unit; (d)γ-(Ni-Mex-Mey-Mez ) unit
Table 3 Valence electron structures of Ni-Cr -Mo- Cu corrosion resistant alloy
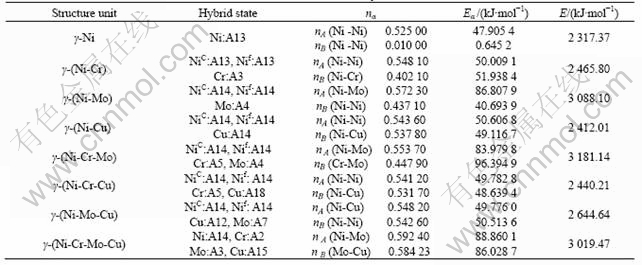
Table 4 Valence electron structures of 1Cr18Ni9Ti stainless steel
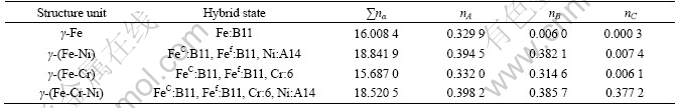
First, from Tables 3 and 4, the bond strength nA of both pure nickel and Ni-Cr-Mo-Cu alloy is much larger than that of 1Cr18Ni9Ti (about over 52%, their average values are 0.553 1 and 0.363 6, respectively).
Second, nA increases with the addition of element Cr, Mo and Cu to γ-Ni (the largest value reaches 0.592 4),
and the bond energy EA from 47.905 4 kJ/mol to 88.860 1 kJ/mol respectively. Especially, increment of the bond energy of the constructed Ni-Mo bonds in Mo-containing units attains 75%-85% comparable to Ni-Ni bond of γ-Ni. And the main bond network and its co-valence bond strength of Ni-Cr-Mo-Cu alloy are enhanced greatly, which causes the effects of solution strengthening. As a result, the strength of the tested Ni-Cr-Mo-Cu alloy (570-700 MPa) is not only higher than that of as-solution 1Cr18Ni9Ti austenitic stainless steel, but also much higher (about 30%) than that of pure nickel (about 450 MPa).
In fact, the corrosion of metals in solution belongs to the electrochemical corrosion, which always matches with an anode reaction, metal as an anode solutes and changes into positive ions or compound relative to the loss of material. Meanwhile, the electrons produced in the anode reaction will flow through the metal and be used up in a cathodic reaction. Therefore, the corrosion process and its reaction rate of metal mainly depend on the extent of metal ionization and the flow resistance of charged micro-particles.
For the present Ni-alloy, on the one hand, with the addition of Cr, Mo and Cu, the binding energy E (about 37% for γ-(Ni-Cr-Mo) units over γ-Ni unit) and the strongest bond strength nA all increase, these leading to the higher stability of metallic system, the larger binding to metal atoms and the more difficulty of metallic ionizing process. On the other hand, with the enhancement of the main bond network, bond energy and binding energy, the vibrating frequency and restoring force of atomic bonds will all rise greatly[12] and the “empty orbit” space of unsaturated fractional co-valence bonds by resonance[10] will be shortened, which causes the larger obstruction against the electron flow from anode to cathode in corrosion process. These factors will improve the corrosion resistant ability of Ni-Cr-Mo-Cu alloy. The present tested alloy shows much low corrosive rate either in oxidizing solutions or in reducing solutions, especially in FeCl3 and HCl solutions, about 2-3 orders of magnitude as high as 1Cr18Ni9Ti austenitic stainless steel for its corrosive resistance.
5 Conclusions
1) The valence electron structures were built and the relative parameters nA, nB, EA, EB and E were calculated for newly synthesized Ni-Cr-Mo-Cu corrosion resistant alloy.
2) With the addition of element Cr, Mo and Cu to nickel, the bond strength nA and bond energy EA of the strongest bond all increase obviously, which causes the larger solution strengthening effects (about 30% increase in σb).
3) The solutions of Cr, Mo and Cu can reinforce the main bond network and binding energy, its average value of nA 52% over 1Cr18Ni9Ti stainless steel and Ni-Mo bond energy of Ni-Cr-Mo-Cu alloy 80% over Ni-Ni bond energy of pure nickel, which will make the ionization of metal atoms in corrosion solution and the flow of electrons form anode to cathod more difficult. As a result, the present Ni-Cr-Mo-Cu alloy has excellent corrosion resistant ability, about 2-3 orders of magnitude as high as 1Cr18Ni9Ti in highly aggressive solutions.
References
[1] YANG Rui-cheng. Characteristics and research trends of high performance Ni-base corrosion resistant alloys[J]. Materials Review, 2001, 15(11): 21-23. (in Chinese)
[2] REBAK R B. Nickel alloys for corrosive environments[J]. Advanced Materials and Processes, 2000, 157(2): 37-42.
[3] MACHET A, GALTAYRIES A, MARCUS P. XPS study of oxides formed on nickel-base alloys in high-temperature and high-pressure water[J]. Surface and Interface Analysis, 2003, 34: 197-200.
[4] STEPHEN E, ZIEMNIA K, HANSON M. Corrosion behavior of NiCrFe alloy 600 in high temperature, hydrogenated water[J]. Corrosion Science, 2006, 48: 498–521.
[5] STEPHEN E Z. Corrosion behavior of NiCrMo Alloy 625 in high temperature, hydrogenated water[J]. Corrosion Science, 2003, 45(9): 1595-1618.
[6] CHENG Xue-qun, LI Xiao-gang, DU Cui-wei. Corrosion behavior of stainless steel and nickel based alloy in acetic acid solution[J]. Journal of Chinese Society for Corrosion and Protection, 2006, 26(2): 70-74. (in Chinese)
[7] GHOSHA S K, DEYB G K, DUSANE R O. Improved pitting corrosion behaviour of electrodeposited nanocrystalline Ni–Cu alloys in 3.0% NaCl solution[J]. Journal of Alloys and Compounds, 2006, 426: 235–243.
[8] YANG Fang-zu, HUANG Bing-qiang, HUANG Ling. Electrochemical corrosion behavior of nickel-base alloy coatings in hydrochloric acid[J]. Plating and Finishing, 2005, 27(4): 1-4. (in Chinese)
[9] XU Jiang, XIE Xi-shan, XU Zhong,. Investigation on multi-element Ni-Cr-Mo-Cu alloying layer by double glow plasma alloying technique[J]. Materials Chemistry and Physics, 2005, 92(3): 340-347.
[10] ZHANG Rui-lin. The empirical electron theory of solids and molecules(EET)[M]. Jilin: Jilin Science and Technology Press, 1993: 231-270. (in Chinese)
[11] WANG Kai-xuan; YANG Rui-cheng. Development of versatile Ni-base corrosion resistant alloy[J]. Journal of Lanzhou University of Technology, 2005, 31(6): 28-31. (in Chinese)
[12] YANG Rui-cheng, CHEN Kui. Determination and application of Larson-Miller Parameter for Heat Resistant Steel 12Cr1MoV and 15CrMo[J]. Acta Metallurgica Sinica, 2004, 17(4): 471-476.
Foundation item: Project (SKL05011) supported by the Foundation of State Key Laboratory of Gansu Advanced Non-ferrous Metal Materials, China
Corresponding author: YANG Rui-cheng; Tel: +86-931-2755239; E-mail: yangruicheng@lut.cn
(Edited by YANG You-ping)


