Trans. Nonferrous Met. Soc. China 30(2020) 1111-1123
Simultaneous extraction of gold and zinc from refractory carbonaceous gold ore by chlorination roasting process
Hong-jun WANG1, Ya-li FENG1, Hao-ran LI2, Jin-xing KANG1
1. School of Civil and Resources Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
Received 27 May 2019; accepted 17 February 2020
Abstract:
A novel process based on chlorination roasting was proposed to simultaneously recover gold and zinc from refractory carbonaceous gold ore by using NaCl as chlorination agent. The effects of roasting temperature, roasting time and NaCl content on the volatilization rates of gold and zinc were investigated. The reaction mechanism and the phase transition process were also analyzed by means of SEM, EDS and XRD. The results demonstrated that under the optimal conditions of NaCl content of 10%, roasting temperature of 800 °C, roasting time of 4 h and gas flow rate of 1 L/min, the rates of gold and zinc were 92% and 92.56%, respectively. During low-temperature chlorination roasting stage, a certain content of sulfur was beneficial to the chlorination reactions of gold and zinc; and during high-temperature chlorination roasting stage, the crystal structure of vanadium-bearing mica was destroyed, and the vanadium-containing oxides were beneficial to the chlorinating volatilization of gold and zinc. Eventually, the chlorinated volatiles of gold and zinc could be recovered by alkaline solution.
Key words:
refractory carbonaceous gold ore; chlorination roasting; thermodynamic calculation; gold; zinc;
1 Introduction
With progressive exhaustion of non-refractory gold ores, refractory gold ores have become main materials for the production of gold, and nearly one third of gold in the world comes from refractory gold ores [1-4]. As a kind of refractory gold ore resources, carbonaceous gold ore containing carbonaceous matter and sulfide minerals is generally considered as double-refractory [4,5]. Its processing is relatively complicated due to “preg-robbing” and the inhibition of the dissolution of gold by carbonaceous matter [6]. In order to recover gold from gold ores, various leaching processes have been proposed, and the leaching reagents mainly include cyanide [2,3], thiourea [7,8] and thiosulfate [9-11]. Cyanide leaching is restricted because of increasing concerns regarding the extremely high toxicity of cyanide which can easily cause health problems and environmental pollution [11,12]. Additionally, it cannot be used for directly extracting gold from the refractory gold ores containing both sulfur and carbonaceous matter [13,14]. Thiourea is also a suspected carcinogen [15]. In addition, the expensive price, high consumption and instability of thiourea and thiosulfate prevent their wide application in gold recovery [11,15]. Biotechnological leaching is considered as an alternative for the extraction of gold from carbonaceous gold ore due to its low cost and environmental impact compared with conventional methods [1,16,17]. However, the main drawback of the biotechnological leaching process is inefficient and too sensitive to operating conditions as well as its relatively slow process as compared with other leaching processes [10].
Moreover, except for containing gold, the carbonaceous gold ore in this study contains zinc. Recently, more than 80% of metal zinc in the world is recovered from zinc sulfide concentrates by the roasting-leaching-electrowinning process [18-21]. The oxidation roasting process can oxidize sulfur and remove organic carbon, and also can convert zinc sulfide to oxide structure which is easier to dissolve in dilute sulfuric acid solution [22-24]. However, a significant fraction of zinc oxide tends to react with iron impurities to form zinc ferrite (ZnFe2O4), which is insoluble in traditional acid leaching process and makes the recovery of zinc difficult, thereby resulting in serious loss of zinc [20,21,24,25].
Therefore, a novel process should be designed for simultaneously recovering gold and zinc from the refractory carbonaceous gold ore. In the whole process, the recovery of gold should be ensured firstly due to its considerable high economic value, and simultaneously the recovery of zinc should also be considered, which increase the difficulty of the whole process. OJEDA et al [26] investigated the feasibility of the extraction of gold from alluvial material by means of chlorination process, using chlorine as a reactive agent. And the highest recovery of gold was 98.23% at 873 K for 1 h. JAAFAR et al [27] reported that high temperature chlorination roasting could selectively remove Zn from steelmaking dust by using ammonium chloride as chlorination reagent. The chlorination roasting process has high selectivity due to different thermodynamic stabilities of different metal chlorides [28]. Additionally, the chlorination roasting process is also presented from environmentally acceptable aspect [29]. Considering the fact that the carbonaceous gold ore contains vanadium, calcium chloride can react with vanadium to form insoluble calcium vanadate, sodium chloride can react with vanadium to generate soluble sodium vanadate, and ammonium chloride is easy to decompose during low temperature roasting process. Therefore, sodium chloride was chosen as chlorination agent in the process.
Therefore, in this study, one-step chlorination roasting method was proposed to simultaneously extract gold and zinc from a refractory carbonaceous gold ore. According to our knowledge, it is first time to simultaneously extract gold and zinc by one-step chlorination roasting. This work focused on the feasibility and mechanism of one-step chlorination roasting for simultaneous extraction of gold and zinc from the refractory carbonaceous gold ore, which aimed to provide new insights for comprehensive utilization of the refractory carbonaceous gold ore containing multiple valuable elements.
2 Experimental
2.1 Materials and products identification
Chemical compositions of the sample were determined by X-ray fluorescence spectrometry (XRF, Shimadzu XRF-1800). Phase compositions of the solid roasting products were investigated by X-ray powder diffraction (XRD, Rigaku, D/max 2500VB+; Cu Kα radiation, 40 kV, 250 mA). Morphology study, element distribution and chemical composition analyses were conducted by scanning electron microscopy (SEM, JSM-7001F, JEOL, Japan) equipped with energy disperse X-ray spectrometry (EDS, Inca X-Max, Oxford Instruments, America). In the experiments, the roasting equipment was a tubular muffle furnace which was produced by Beijing Fulemon High- temperature Technology Co., Ltd. All chemical reagents used in this study were of analytical grade and provided by Shanghai Science and Technology Co., Ltd., which were directly used without further purification. Deionized water was used in all experiments.
The refractory carbonaceous gold ore used in this study was collected from a mineral processing plant located in Shanxi province, China. The pure pyrite was provided by Institute of Process Engineering, Chinese Academy of Science. The chemical composition of the refractory carbonaceous gold ore shown in Table 1 indicates that the main valuable compositions in the sample used in the study are gold, vanadium and zinc; the contents of gold, vanadium and zinc are 21.11 g/t, 1.05 wt.% and 0.54 wt.%, respectively; and the total contents of carbon and sulfur are 11.00 wt.% and 3.01 wt.%, respectively. The XRD patterns and SEM-EDS images of the raw ore are shown in Fig. 1 and Fig. 2, respectively. Combined with the results of XRF, XRD and SEM-EDS, the carbonaceous gold ore is composed of quartz, sphalerite, mica and carbonaceous mineral; vanadium in the carbonaceous gold ore mainly exists in muscovite, and zinc mainly exists in the form of sphalerite. It can be concluded from the above results that this type of carbonaceous gold ore cannot be directly leached by conventional cyanidation. Therefore, a novel extraction process should be proposed to recover gold and other valuable elements in the refractory carbonaceous gold ore. The chemical composition of the pure pyrite sample used as sulfur additive in the experiment is shown in Table 2. Combined with the results of XRF, there are no gold, vanadium and zinc in the pure pyrite sample, and the main mineral is pyrite.
Table 1 Chemical compositions of carbonaceous gold ore (wt.%)

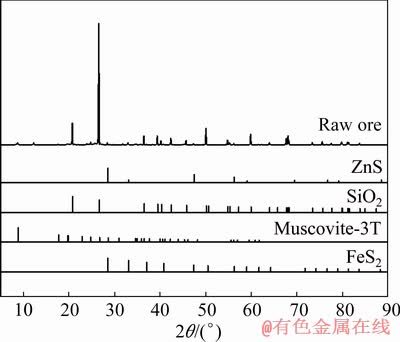
Fig. 1 XRD patterns of carbonaceous gold ore
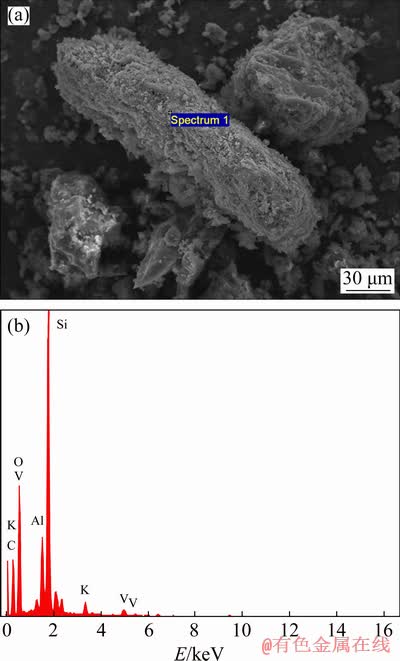
Fig. 2 SEM image (a) and EDS spectrum (b) of carbonaceous gold ore
Table 2 Chemical composition of pure pyrite (wt.%)

2.2 Experimental methods
2.2.1 Experimental procedure
The experiments for the extraction of gold and zinc from the refractory carbonaceous gold ore were carried out by a simple one-step chlorination roasting. The flow sheet is shown in Fig. 3(a). The effects of sulfur content on the extraction of gold and zinc were also investigated, and the flow sheet of verification experiment is shown in Fig. 3(b). In the chlorination roasting process, sodium chloride was used as chlorination agent, and pyrite was served as sulfur additive. The influence of chlorination roasting parameters such as roasting temperature, roasting time and NaCl content as well as S content was studied, as shown in Table 3. Here, the NaCl content was defined as the mass fraction of NaCl to sample, and the S content was defined as the mass fraction of sulfur in the pure pyrite to sample.
2.2.2 Chlorination roasting of carbonaceous gold ore

Fig. 3 Flow sheet of simultaneous extraction of gold and zinc by chlorination roasting (a), and flow sheet of verification experiment of sulfur content on extraction of gold and zinc (b)
Table 3 Experimental conditions used in each operation


Fig. 4 Schematic diagram of equipment for roasting experiment
All the roasting experiments were carried out in the experimental apparatus, as shown in Fig. 4. The chlorinated volatile gases containing gold and zinc were adsorbed by excessive sodium hydroxide solution (0.2 mol/L) [30], and eventually gold and zinc were recovered in the form of precipitates. In a typical experimental procedure, the tube furnace was heated to the needed temperature at a certain heating rate. Subsequently, 20 g of sample and a certain amount of NaCl were placed in quartz porcelain boat and quickly transferred into the intermediate position of the tube furnace. And the gas flow rate was fixed at 1 L/min. Then, the chlorination roasting experiments were run for a preset time at the required roasting temperature. After finishing the roasting process, the air continued to be forced into the tube furnace until the roasting temperature naturally dropped to 100 °C. At last, the as-obtained roasted residue was taken out from the tube furnace. The pH value of NaOH solution was adjusted until no more precipitate was produced, and then the resultant precipitate was filtered and dried in the drying oven. For every experimental condition, two groups of experiments were carried out. One group of roasted residue and precipitate was used to characterize phase compositions and morphology observation by XRD and SEM. The other roasted residue was used to analyze the contents of gold and zinc. The volatilization rates of gold and zinc were calculated based on the contents of gold and zinc in the roasted residue, and the corresponding equation could be expressed as follows:
 (1)
(1)
where γ1 was gold (zinc) volatilization rate, %; m represented the mass of the sample, g; m1 was mass of the roasted residue, g; θ was gold (zinc) content in the sample, g/t (%); and θ1 was gold (zinc) content in the roasted residue, g/t (%).
2.2.3 Verification experiment of sulfur content on chlorinating volatilization of gold and zinc
All the roasting experiments were performed in the above experimental apparatus. The gold- and zinc-bearing chlorinated volatile gases were adsorbed by excessive sodium hydroxide solution (0.2 mol/L). In all roasting experiments, the tube furnace was heated to 600 °C. Subsequently, 20 g of sample was placed in quartz porcelain boat and quickly transferred into the intermediate position of the tube furnace. The gas flow rate was controlled at 1 L/min. In order to completely remove sulfur from the carbonaceous gold ore, the roasting experiments were run for 2 h. When the roasting temperature dropped to 100 °C, the roasting material was taken out from the tube furnace and then mixed with 10% NaCl, 11% C and a certain amount of pyrite. Here, the addition of C was to eliminate its interference in the whole roasting process. Subsequently, when the tube furnace was heated to 600 °C, the above mixture was placed in quartz porcelain boat and quickly transferred into the intermediate position of the tube furnace. The air flow rate was also fixed at 1 L/min. The roasting experiments were run at 600 °C for 4 h. After finishing the roasting process, the air continued to be forced into the tube furnace until the roasting temperature dropped to 100 °C, and then the roasted residue was taken out from the tube furnace. The pH value of NaOH solution was adjusted until no more precipitate was produced, and then the resultant precipitate was filtered and dried in drying oven. The volatilization rates of gold and zinc in the carbonaceous gold ore were calculated based on Eq. (1).
3 Results and discussion
3.1 Reaction principle and thermodynamic analysis
As a principal criterion, Gibbs free energy change (ΔG) is often used to judge whether a chemical reaction can occur, and a reaction is likely to occur when its ΔG is negative [31]. During the roasting process, sodium chloride can be dissociated to generate chlorine under the action of oxygen. The roasting dissociation mechanism of sodium chloride in the presence of oxygen can be explained by Eqs. (2)-(8):
NaCl+1/4O2(g)=1/2Na2O+1/2Cl2(g) (2)
NaCl+1/4O2(g)+1/2V2O5=NaVO3+1/2Cl2(g) (3)
NaCl+3/4O2(g)+1/2V2O3=NaVO3+1/2Cl2(g) (4)
NaCl+19/16O2(g)+1/4FeS2=1/2Na2SO4+1/2Cl2(g)+1/8Fe2O3 (5)
NaCl+1/2O2(g)+1/2O2=1/2Na2SO4+1/2Cl2(g) (6)
NaCl+1/4O2(g)+1/2SiO2=1/2Na2SiO3+1/2Cl2(g) (7)
NaCl+5/4O2(g)+1/2ZnS=1/2Na2SO4+1/2Cl2(g)+1/2ZnO (8)
During the chlorination roasting process of carbonaceous gold ore, oxygen and chlorine exist in the reaction system. Therefore, chlorination and oxidation reactions of various phases in the carbonaceous gold ore can occur, and the corresponding reactions as shown in Eqs. (9)-(20):
V2O3+O2(g)=V2O5 (9)
V2O5+3Cl2(g)=2VOCl3+3/2O2(g) (10)
V2O5+3Cl2(g)=2VCl3+5/2O2(g) (11)
FeS2+O2(g)=Fe2O3+SO2(g) (12)
FeS2+3/2Cl2(g)=FeCl3+2S (13)
Fe2O3+3Cl2(g)=2FeCl3+3/2O2(g) (14)
ZnS+3/2O2(g)=ZnO+SO2(g) (15)
ZnO+Cl2(g)=ZnCl2(g)+1/2O2(g) (16)
ZnS+Cl2(g)=ZnCl2(g)+S (17)
S+O2(g)=SO2(g) (18)
Au+3/2Cl2(g)=AuCl3(g) (19)
Au+3/4O2(g)=1/2Au2O3 (20)
The ΔG values of various reactions are calculated by using HSC software, as shown in Fig. 5(a). It can be speculated from Fig. 5(a) that the self-dissolution reaction of NaCl is difficult to occur; and the ΔG values of the dissolution reactions of NaCl in the presence of V2O3, FeS2, ZnS, SO2 and SiO2 are less than that of the self-dissolution reaction of NaCl. Therefore, sulfide minerals and low-valence vanadium oxides are beneficial to the dissolution reactions of NaCl, and the promoting effect on the dissolution of NaCl from high to low is based on the following rule: ZnS > FeS2 > V2O3 > SO2 > V2O5 > SiO2.
The ΔG values of various reactions are calculated by using HSC software, as shown in Fig. 5(b). It can be speculated that ZnS, FeS2 and V2O3 are easily oxidized to ZnO, Fe2O3 and V2O5 compared with the chlorination reactions. The ΔG values of the chlorination reactions of Fe2O3 and V2O5 are positive in the whole roasting temperature range, indicating that the chlorination reactions are difficult to occur, and vanadium is more easily converted into soluble vanadate. In chlorination roasting process, ZnO is easy to be chlorinated and simultaneously generate zinc chloride with the increase of roasting temperature. And compared with oxidation reaction, gold is easier to react with chlorine to generate gold chloride.
The predominant phase diagrams of various systems at 800 °C are obtained by using HSC software, as shown in Fig. 6. It can be clearly observed that for every system, the diagram is divided into areas of stability of various phases for a certain element. Under the conditions of high Cl2 partial pressure and low O2 partial pressure, the main stable phases of Zn, Fe, V and Au tend to be volatile ZnCl2, FeCl3, VCl3 and AuCl3; and under the same condition, such as p(Cl2)=1×10-4 Pa and p(O2)=1×10-5 Pa, Zn tends to be volatile chloride compared with Fe, V and Au. Therefore, it can be speculated from the thermodynamic assessment that ZnCl2 is more easily formed than FeCl3, VCl3 and AuCl3.

Fig. 5 Gibbs free energy changes for dissolution reactions of sodium chloride (a) and for chlorination and oxidation reactions of various phases in carbonaceous gold ore (b)

Fig. 6 Predominant phase diagrams of various systems at 800 °C
3.2 Chlorinating volatilization
3.2.1 Effect of roasting temperature
It is common that there are some differences between the thermodynamic analysis and practical process because of dynamic influence. Therefore, it is necessary to study the chlorination reactions of gold and zinc by chlorination roasting experiments, in spite of positive results shown in terms of thermodynamics. The effects of the roasting temperature on the volatilization rates of gold and zinc were therefore investigated with the roasting temperature ranging from 400 to 900 °C under the conditions of NaCl content of 10%, roasting time of 4 h and air flow rate of 1 L/min. The experimental data are given in Fig. 7.
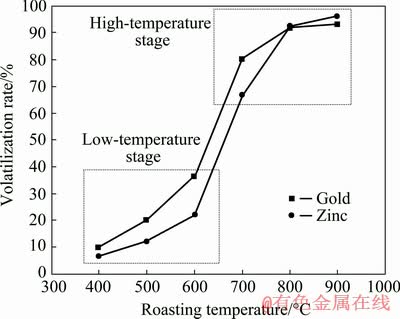
Fig. 7 Effect of roasting temperature on volatilization rates of gold and zinc
It can be clearly observed from Fig. 7 that the volatilization rates of gold and zinc increase with the increase of the roasting temperature, indicating that they are strongly dependent on the roasting temperature, and they have similar change trend. When the roasting temperature increases from 400 to 600 °C, the volatilization rates of gold and zinc increase slowly, and the values of gold and zinc increase from 10% to 36.36% and from 6.73% to 22.11%, respectively. Thermodynamic analysis demonstrates that compared with chlorination reaction, zinc sulfide is more easily oxidized to zinc oxide. Therefore, in the chlorinating volatilization process, zinc sulfide may be firstly oxidized to zinc oxide, and then the resultant zinc oxide is chlorinated to zinc chloride. During the low temperature roasting stage of 400-600 °C, the low volatilization rate of zinc may be attributed to the fact that in the roasting temperature range, zinc sulfide is mainly oxidized, and simultaneously the low temperature roasting results in a relatively low oxidation rate for zinc sulfide. Therefore, a small fraction of zinc can be chlorinated in the low roasting temperature stage. Because vanadium mainly exists in mica, the crystal structure of mica may not be destroyed during low temperature chlorination roasting, resulting in the fact that the vanadium encapsulated in mica cannot contact with sodium chloride and correspondingly less chlorine is produced in the system [32,33], thereby leading to relatively low chlorination rates of gold and zinc.
When the roasting temperature increases from 600 to 800 °C, the volatilization rates of gold and zinc increase obviously. The values of gold and zinc are increased by 55.64% and 70.45%, respectively. In the high temperature chlorination roasting stage of 700-900 °C, the relatively high volatilization rates of gold and zinc may be attributed to the fact that with increasing roasting temperature, the crystal structure of mica is destroyed, and the vanadium encapsulated in mica can contact with sodium chloride. Subsequently, the oxidation rate of zinc sulfide is increased, and correspondingly more chlorine is produced in the system, thereby resulting in relatively high chlorination rates of gold and zinc. Therefore, the optimal roasting temperature is determined to be 800 °C.
3.2.2 Effect of roasting time
Roasting time is an important parameter for the chlorination roasting process. A series of experiments were performed at different roasting time under the conditions of NaCl content of 10%, roasting temperature of 800 °C and air flow rate of 1 L/min. The results are shown in Fig. 8.
It can be clearly seen from Fig. 8 that with the increase of roasting time, the gold volatilization rate increases obviously but the zinc volatilization rate increases slowly. For example, when the roasting time is 1 h, the gold volatilization rate of 23.78% is achieved, while the zinc volatilization rate of 78.14% is obtained. When the roasting time is 4 h, the volatilization rates of gold and zinc are 92.00% and 92.56%, respectively. This is probably because with the increase of roasting time, the crystal structure of mica is destroyed continuously, therefore the oxidation rate of zinc sulfide increases, and simultaneously the chlorine content in the reaction system increases, resulting in the increases of chlorinating volatilization rates of gold and zinc. This indicates that the chlorination reaction of zinc is easier to occur compared with that of gold [34,35]. Therefore, the optimal roasting time is determined to be 4 h.

Fig. 8 Effect of roasting time on volatilization rates of gold and zinc
3.2.3 Effect of NaCl content
The effects of NaCl content on the volatilization rates of gold and zinc were examined at the NaCl content ranging from 2.5% to 15% under the conditions of roasting temperature of 800 °C, roasting time of 4 h and air flow rate of 1 L/min. The results are shown in Fig. 9.

Fig. 9 Effect of NaCl content on volatilization rates of gold and zinc
It can be clearly observed from Fig. 9 that the NaCl content has a significant influence on the volatilization rates of gold and zinc. When the NaCl content increases from 2.5% to 10%, the volatilization rates of gold and zinc increase from 69.00% to 91.56% and from 67.12% to 92.56%, respectively. When the NaCl content increases further the changes in the values for gold and zinc are not obvious. Therefore, the optimum NaCl contnet is determined to be 10%.
3.3 SEM, EDS and XRD results of roasted residue and volatile precipitate
3.3.1 SEM, EDS and XRD results of roasted residue
Figure 10 shows the SEM images and EDS spectra of roasted residues obtained at different roasting temperatures under the conditions of NaCl content of 10%, roasting time of 4 h and air flow rate of 1 L/min.
Figures 10(a1, a2) show that the crystal structure of mica in the roasted residue obtained at 600 °C is not destroyed, indicating that the vanadium in the mica is not oxidized. Combined with the roasting temperature experiments of Fig. 7 and correspondingly thermodynamic calculation, in the low-temperature chlorination roasting stage, the chlorinating volatilization of gold and zinc is mainly ascribed to the action of sulfur in FeS2 and ZnS, which facilitate the decomposition reaction of sodium chloride, thereby promoting the chlorinating volatilization of gold and zinc.
Combined with Figs. 10(b1, b2, c1, c2) and Fig. 11, the bright region of the roasted residue obtained at 700 °C is rich in K, Na, Si, Al and O, and the molar ratio of the roasted residue is same as that of sodium potassium feldspar, indicating that the crystal structure of the mica in the roasted residue is destroyed and sodium potassium feldspar may be formed. High temperature chlorination roasting can make the dense ore particles loose and porous, and simultaneously the specific surface area of the roasted residue increases. Combined with Fig. 7 and the corresponding thermodynamic calculation, it can be concluded that when the roasting temperature is above 700 °C, V2O3, FeS2 and ZnS can facilitate the decomposition reaction of sodium chloride, thereby promoting the chlorinating volatilization of gold and zinc.
3.3.2 Verification of gold volatilization by using sulfur

Fig. 10 SEM images (a1, b1, c1) and EDS spectra (a2, b2, c2) of roasted residues at different roasting temperatures

Fig. 11 XRD pattern of residue at roasting temperature of 800 °C
In order to verify the experimental results and explore the effect of sulfur content on the chlorinating volatilization of gold and zinc in the low-temperature roasting stage, the verification experiments of sulfur content were carried out, and the experimental results are shown in Fig. 12.

Fig. 12 Verification experimental results of sulfur content (Pre-desulfurization for 2 h and then roasting under conditions of NaCl 10%, roasting temperature 600 °C, roasting time 4 h and air flow rate 1 L/min)
It can be clearly seen that sulfur content has a more significant influence on the gold volatilization rate as compared with the zinc volatilization rate. With the increase of sulfur content, the volatilization rates of gold and zinc increase until they reach the maximum values at sulfur content of 3%, and then decline when sulfur content increases further. The maximum volatilization rates of gold and zinc are 37.13% and 22.34%, respectively. In the all validation experiments, the sulfur in the carbonaceous gold ore was removed by roasting at 600 °C for 2 h, and subsequently pyrite was added as sulfur additive. The above results indicate that the sulfur in the carbonaceous gold ore plays an extremely important role in the chlorinating volatilization of gold and zinc, and the optimal sulfur content is determined to be 3%. It is worth mentioning that the sulfur content in the carbonaceous gold ore is also 3%.
3.3.3 Characteristics of volatile precipitates at 800 °C
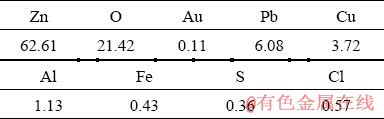
Combined with the results of Figs. 13 and 14, the chlorinated volatiles of gold and zinc can be recovered by alkaline solution, and the chlorinated volatile of zinc is precipitated in the form of Zn5(OH)6(CO3)2, and the gold volatile is precipitated along with the zinc. At the same time, the results of multi-element analysis of volatile precipitate obtained by chlorination roasting at 800 °C and adsorbed by alkali liquor are shown in Table 4. The precipitation mechanisms of gold and zinc need to be further studied in the future.

Fig. 13 EDS image (a) and elemental distribution (b-d) of volatile precipitates at 800 °C

Fig. 14 XRD patterns of volatile precipitates at 800 °C
Table 4 Results of multi-element analysis of volatile precipitates obtained at 800 °C (wt. %)
4 Conclusions
(1) The thermodynamic calculation results demonstrated that gold and zinc were easy to be chlorinated; chlorination agent NaCl could realize the chlorinating volatilization of gold and zinc in the carbonaceous gold ore under the action of O2, V2O3, FeS2 and ZnS, which provided a theoretical basis for the chlorinating volatilization of gold and zinc.
(2) During the low-temperature chlorination roasting, the sulfur in FeS2 and ZnS could promote the dissolution reaction of sodium chloride, which was beneficial to the chlorinating volatilization of gold and zinc. During the high temperature chlorination roasting, the crystal structure of mica was destroyed, and the dissolution reactions of sodium chloride could be promoted by V2O3, FeS2 and ZnS.
(3) The chlorinated volatiles of gold and zinc could be recovered by alkaline solution. The zinc volatile was precipitated in the form of Zn5(OH)6(CO3)2 and the gold volatile was precipitated along with zinc.
(4) The chlorination reactions of gold and zinc could be improved by increasing roasting temperature, roasting time and NaCl content. Under the optimal conditions of NaCl content of 10%, roasting temperature of 800 °C, roasting time of 4 h and air flow rate of 1 L/min, the volatilization rates of gold and zinc were 92.00% and 92.56%, respectively.
References
[1] YANG Hong-ying, LIU Qian, SONG Xiang-ling, DONG Jin-kui. Research status of carbonaceous matter in carbonaceous gold ores and bio-oxidation pretreatment [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3405-3411.
[2] TAN H, FENG D, LUKEY G, DEVENTER J. The behaviour of carbonaceous matter in cyanide leaching of gold [J]. Hydrometallurgy, 2005, 78: 226-235.
[3] GUO Xue-yi, XIN Yun-tao, WANG HAO, TIAN Qing-hua. Mineralogical characterization and pretreatment for antimony extraction by ozone of antimony-bearing refractory gold concentrates [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1888-1895.
[4] HE Yu-chen, XU Zhen-ming. Recycling gold and copper from waste printed circuit boards using chlorination process [J]. RSC Advances, 2015, 5: 8957-8964.
[5] MARCHEVSKY N, BARROSOQUIROGA M, GIAVENO A, DONATI E. Microbial oxidation of refractory gold sulfide concentrate by a native consortium [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1143-1149.
[6] LIU Qian, YANG Hong-ying, TONG Lin-lin, JIN Zhen-nan, SAND W. Fungal degradation of elemental carbon in carbonaceous gold ore [J]. Hydrometallurgy, 2016, 160: 90-97.
[7] YANG Xi-yun, MOATS M, MILLER J, WANG Xu-ming, SHI Xi-chang, XU Hui. Thiourea-thiocyanate leaching system for gold [J]. Hydrometallurgy , 2011, 106: 58-63.
[8] LI Jing-ying, XU Xiu-li, LIU Wen-quan. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones [J]. Waste Management, 2012, 32: 1209-1212.
[9] OFORISARPONG G, OSSEOASARE K. Preg-robbing of gold from cyanide and non-cyanide complexes: Effect of fungi pretreatment of carbonaceous matter [J]. International Journal of Mineral Processing, 2013, 119: 27-33.
[10] BAS A, SAFIZADEH F, ZHANG W, GHALI E, CHOI Y. Active and passive behaviors of gold in cyanide solutions [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3442-3453.
[11] LEI Chang, YAN Bo, CHEN Tao, WANG Xiao-liang, XIAO Xian-ming. Silver leaching and recovery of valuable metals from magnetic tailings using chloride leaching [J]. Journal of Cleaner Production, 2018, 181: 408-415.
[12] XU Bin, YANG Yong-bin, LI Qian, JIANG Tao, LIU Shi-qian, LI Guang-hui. The development of an environmentally friendly leaching process of a high C, As and Sb bearing sulfide gold concentrate [J]. Minerals Engineering, 2016, 89: 138-147.
[13] TAN H, FENG D, VANDEVENTER J, LUKEY G. Effect of contaminant carbonaceous matter on the sorption of gold by pyrite [J]. International Journal of Mineral Processing, 2005, 77: 123-138.
[14] HEDIAZI F, MONHEMIUS A. Industrial application of ammonia-assisted cyanide leaching for copper-gold ores [J]. Minerals Engineering, 2018, 126: 123-129.
[15] PANI B, SWAIN S, PRADHAN S, SINGH U. Enhancement of structural and optoelectronic properties of vacuum processed Cu2ZnSnS4 thin film by thiourea treatment [J]. Journal of Alloys and Compounds, 2017, 708: 181-186.
[16] SYED S, Recovery of gold from secondary sources- A review [J]. Hydrometallurgy, 2010, 96: 30-51.
[17] GUO Yu-jie, GUO Xue, WU Hai-yan, LI Shou-peng, WANG Guo-hua, LIU Xin-xing, QIU Guan-zhou, WANG Dian-zuo. A novel bio-oxidation and two-step thiourea leaching method applied to a refractory gold concentrate [J]. Hydrometallurgy, 2017, 171: 213-221.
[18] YAN Huan, CHAI Li-yuan, PENG Bing, LI Mi, PENG Ning, HOU Dong-ke. A novel method to recover zinc and iron from zinc leaching residue [J]. Minerals Engineering, 2014, 55: 103-110.
[19] XU Zhi-feng, JIANG Qing-zheng, WANG Cheng-yan. Atmospheric oxygen-rich direct leaching behavior of zinc sulphide concentrate [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3780-3787.
[20] GENG Shu-hua, LI Guang-shi, ZHAO Yong, CHENG Hong-wei, LU Yi, LU Xiong-gang, XU Qian. Extraction of valuable metals from low nickel matte by calcified roasting-acid leaching process [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 2202-2212.
[21] LI Yan-chun, LIU Hui, PENG Bing, MIN Xiao-bo, HU Min, PENG Ning, YUANG Ying-zhen, LEI Jie. Study on separating of zinc and iron from zinc leaching residues by roasting with ammonium sulphate [J]. Hydrometallurgy, 2015, 158: 42-48.
[22] NANTHAKUMAR B, PICKLES C, KELEBEK S. Microwave pretreatment of a double refractory gold ore [J]. Minerals Engineering, 2007, 20: 1109-1119.
[23] TONG Xiong, LV Jin-fang, ZHENG Yong-xing, HUANG Lin-yun. DFT study on the interaction between S2 and zincite (101-0) surface [J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 88: 18-24.
[24] YAN Qun-xuan, LI Xin-hai, WANG Zhi-xing, WANG Jie-xi, GUO hua-jun, HU Qi-yang, PENG Wen-jie, WU Xi-fei. Extraction of lithium from lepidolite using chlorination roasting–water leaching process [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 1753-1759.
[25] LI Hao-yu, MA Ai-yuan, SRINIVASAKANNAN C, ZHANG Li-bo, LI Shi-wei, YIN Shao-hua. Investigation on the recovery of gold and silver from cyanide tailings using chlorination roasting process [J]. Journal of Alloys and Compounds, 2018, 763: 241-249.
[26] OJEDA M, PERINO E, RUIZ M. Gold extraction by chlorination using a pyrometallurgical process [J]. Minerals Engineering, 2009, 22: 409-411.
[27] JAAFAR I, GRIFFITHS A, HOPKINS A, STEER J, GRIFFITHS M, SAPSFORD D. An evaluation of chlorination for the removal of zinc from steelmaking dusts [J]. Minerals Engineering, 2011, 24: 1028-1030.
[28] MURASE K, NISHIKAWA K, OZAKI T, MACHIDA K, ADSCHI G, SUDA T. Recovery of vanadium, nickel and magnesium from a fly ash of bitumen-in-water emulsion by chlorination and chemical transport [J]. Journal of Alloys and Compounds, 1998, 264: 151-156.
[29] BROCCHI E, NAVARRO R, MOURA F. A chemical thermodynamics review applied to V2O5 chlorination[J]. Thermochimica Acta, 2013, 559: 1-16.
[30] SHEN Xiao-yi, SHAO Hong-mei, GU Hui-min, CHEN Bing, ZHAI Yu-chun, MA Pei-hua. Reaction mechanism of roasting Zn2SiO4 using NaOH [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 1878-1886.
[31] HAN Jun-wei, LIU Wei, QIN Wen-qing, YANG Kang, WANG Da-wei, LUO Hong-lin. Innovative methodology for comprehensive utilization of high iron bearing zinc calcine [J]. Separation and Purification Technology, 2015, 154: 263-270.
[32] WANG Hong-jun, FENG Ya-li, LI Hao-ran, KANG Jin-xing. The separation of gold and vanadium in carbonaceous gold ore by one-step roasting method [J]. Powder Technology, 2019, 355: 191-200.
[33] LI Hao-yu, ZHANG Li-bo, KOPPALA S, MA Ai-yuan, PENG Jin-hui, LI Shi-wei, YIN Shao-hua. Extraction of gold and silver in the selective chlorination roasting process of cyanidation tailing [J]. Separation Science and Technology, 2018, 53: 458-466.
[34] HASHEMAZDEHFINI M, FICERIOVA J, ABKHOSHK E, SHAHRAKI B. Effect of mechanical activation on thiosulfate leaching of gold from complex sulfide concentrate [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2744-2751.
[35] DANG Hui, WANG Ben-feng, CHANG Zhi-dong , WU Xue, FENG Jing-ge, ZHOU Hua-lei, LI Wen-jun, SUN Chang-yan. Recycled lithium from simulated pyrometallurgical slag by chlorination roasting [J]. ACS Sustainable Chemistry & Engineering, 2018, 6: 13160-13167.
氯化焙烧法从难选含碳金矿中同时提取金和锌
王洪君1,冯雅丽1,李浩然2,康金星1
1. 北京科技大学 土木与资源工程学院,北京 100083;
2. 中国科学院过程工程研究所 生化工程国家重点实验室,北京 100190
摘 要:提出一种以NaCl为氯化剂、采用氯化焙烧法从难选含碳质金矿中同时回收金和锌的新工艺。研究焙烧温度、焙烧时间和NaCl含量对金、锌挥发率的影响。采用SEM、EDS和XRD对反应机理和相变过程进行分析。结果表明,在10% NaCl、焙烧温度为800 °C、焙烧时间为4 h、气流速度为1 L/min的最佳条件下,金、锌的挥发率分别为92%和92.56%。在低温氯化焙烧阶段,一定的硫含量有利于金、锌的氯化反应;在高温氯化焙烧阶段,含钒云母的晶体结构被破坏,含钒氧化物有利于金、锌的氯化挥发。最后,金、锌的氯化挥发物可被碱性溶液回收。
关键词:难选碳质金矿;氯化焙烧;热力学计算;金;锌
(Edited by Wei-ping CHEN)
Corresponding author: Ya-li FENG; Tel: +86-13910839080; E-mail: ylfeng126@.126.com
DOI: 10.1016/S1003-6326(20)65282-7
Abstract: A novel process based on chlorination roasting was proposed to simultaneously recover gold and zinc from refractory carbonaceous gold ore by using NaCl as chlorination agent. The effects of roasting temperature, roasting time and NaCl content on the volatilization rates of gold and zinc were investigated. The reaction mechanism and the phase transition process were also analyzed by means of SEM, EDS and XRD. The results demonstrated that under the optimal conditions of NaCl content of 10%, roasting temperature of 800 °C, roasting time of 4 h and gas flow rate of 1 L/min, the rates of gold and zinc were 92% and 92.56%, respectively. During low-temperature chlorination roasting stage, a certain content of sulfur was beneficial to the chlorination reactions of gold and zinc; and during high-temperature chlorination roasting stage, the crystal structure of vanadium-bearing mica was destroyed, and the vanadium-containing oxides were beneficial to the chlorinating volatilization of gold and zinc. Eventually, the chlorinated volatiles of gold and zinc could be recovered by alkaline solution.


