Article ID: 1003-6326(2005)03-0626-05
Inhibition effects of PMA/SbBr3 complex inhibitor on copper and copper-nickel alloy in LiBr solutions
HU Xian-qi(扈显琦), LIANG Cheng-hao(梁成浩), HUANG Nai-bao(黄乃宝)
(School of Chemical Engineering, Dalian University of Technology, Dalian 116012, China)
Abstract:
The effects of PMA/SbBr3 inhibitor on copper and copper-nickel alloy in 55%LiBr solution were investigated by chemical immersion and electrochemical measurements. The results indicate that in boiling 55%LiBr solution containing PMA/SbBr3 inhibitor, corrosion rates of copper and copper-nickel alloy are 67.48μm/a and 38.14μm/a, respectively. Since both anodic and cathodic electrochemical reactions can be inhibited, PMA/SbBr3 belongs to complex inhibitor. PMA has the effect of inhibiting hydrogen evolution and [PMo12O40]3-, the anion of PMA, has a strong oxidizing effect. Sb3+ also shows an oxidizing effect. It may exist in LiBr solutions stably with PMA. Because of the synergistic effect of PMA and Sb3+, a protective film, comprising CuO, Cu2O and Sb, formed on copper and copper-nickel alloy surface may prevent Br- from diffusing to the surface of metals. As a result, the anticorrosion performance of copper and copper-nickel alloy may be improved.
Key words:
copper; copper-nickel alloy; lithium bromide; corrosion; inhibitor CLC;
number: TG174.42 Document code: A
1 INTRODUCTION
To substitute for Freon, LiBr-H2O has been used as working fluid pairs of lithium bromide absorption chiller. Because of the excellent heat transfer performance, copper and copper alloy are often used for making heat exchanger. But lithium bromide solution is a highly corrosive medium for copper, copper alloy, carbon steel, even stainless steel. Some researchers[1] tried to make nickel alloys as lithium bromide absorption chiller. The performances of heat transfer and anticorrosion of nickel alloys are excellent, but they are very expensive. Thus the emphasis of research should be focused on adding inhibitors into lithium bromide solution to decrease corrosion rate by using traditional materials, such as carbon steel, copper and copper alloy[2, 3], as lithium bromide absorption chiller. Usually, the currently used inhibitors have some weaknesses in lithium bromide solution. Li2CrO4 may pollute the environment. Moreover the oxidation value and concentration of chromium are difficult to control. Low concentration of chromium may lead to pitting corrosion[4]. To substitute for Li2CrO4, the application of Li2MoO4 or Na2MoO4 has been studied deeply[5-7]. Na2MoO4 can form protective film on metal surface and avoid pitting corrosion. But researches show that the solubility of Li2MoO4 in LiBr solution is limited, and it is difficult to maintain at an effective concentration. For these reasons, it is very important to look for highly effective inhibitors for lithium bromide absorption chiller. It was reported that heteropoly acid is a polynuclear coordination compound with excellent oxidation-reduction and film-forming characteristics[8]. But as an inhibitor in LiBr solution, it has been scarcely reported. In this paper, the inhibition performance and mechanism of a new type mixed inhibitor composing phosohomolybdic acid(PMA) and SbBr3 on copper and copper alloy in boiling 55% lithium bromide(LiBr) solution are discussed.
2 EXPERIMENTAL
The materials used for measurements were copper and copper-nickel alloy and their chemical compositions are listed in Table 1.Test solution was 55% LiBr solution containing 700mg/L inhibitor composing PMA and SbBr3. Its pH value was controlled at 11.
Rectangular sheet specimens (30mm×20mm×1mm) and square specimens (10mm×10mm×1mm) were used for mass-loss tests and electrochemical measurements, respectively. All specimens were abraded with SiC paper, and rinsed by deionized water and ethanol. 1cm2 working area was exposed to the solution in electrochemical measurements and the other part was covered by Shin-Etsu Silicone.
With a polytetrafluoroethylene(PTFE) cylinder bush(50mm in inner diameter, 65mm in length), stainless steel autoclaves were used in mass-loss tests. The 100cm3 of 55% LiBr solu-
Table 1 Chemical compositions of specimens(mass fraction, %)

tion, containing 700mg/L mixed inhibitor, was deoxygenated for 1h before the experiments. The autoclave was then held at predetermined temperature for 200h in a thermostat(Kosumosu AT-S13). The specimens were cleaned by 5% HCl for 3min at room temperature and then rinsed by deionized water and ethanol. Corrosion rates were calculated from the mass loss of the specimens. Surface state of the copper specimen immersed for 200h was analyzed by electron probe microanalysis(EPMA).
Electrochemical measurements were conducted in a three-electrode system and HA-501 potentiostat/galvanostat. The specimen was immersed in a glass vessel with a platinum counter electrode and a SCE reference electrode. The solution was deoxygenated by nitrogen during the whole process of measurement. When the solution was maintained at boiling state and the blend potential became stable, the polarization curves were measured at a sweep rate of 20mV/min.
3 RESULTS AND DISCUSSION
Corrosion potentials of copper and copper-nickel alloy in boiling 55%LiBr, 55%LiBr+0.10mol/L LiOH +200mg/L Na2MoO4 and 55%LiBr+ PMA/SbBr3 solutions are shown in Fig.1. According to Fig.1, the corrosion potential of copper in 55% LiBr solution is -617mV, which is close to that of copper-nickel alloy, -613mV. While various inhibitors are added into 55%LiBr solution, the corrosion potential moves to positive direction. In 55% LiBr solution with 0.10mol/L LiOH+200mg/L Na2MoO4 , the corrosion potentials of copper and copper-nickel alloy are -579mV and -494mV, respectively. In the solution containing 700mg/L PMA/SbBr3 inhibitor, their corrosion potentials rise to -540mV and -485mV, respectively. The rising value caused by adding PMA/SbBr3 inhibitor is higher than that by adding 0.10mol/L LiOH+200mg/L Na2MoO4 inhibitor. Obviously, the electrochemical performance of copper and copper-nickel alloy may be improved by PMA/SbBr3 inhibitor in 55% LiBr solution.
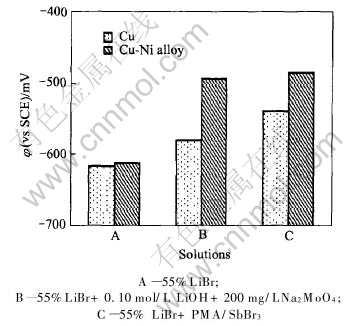
Fig.1 Corrosion potentials of copper and copper-nickel alloy in different solutions
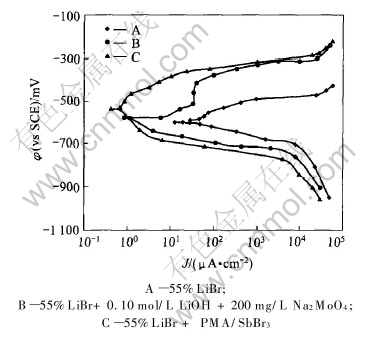
Fig.2 Polarization curves of copper in different boiling solutions
Fig.2 displays the polarization curves of copper in boiling solutions of 55%LiBr, 55%LiBr+PMA/SbBr3 and 55%LiBr+0.10mol/L LiOH+200mg/L Na2MoO4. As seen in Fig.2, adding PMA/SbBr3 inhibitor into 55%LiBr solution causes an obvious left shift of both anodic and cathodic polarization curves and an increase of corrosion potential of copper. Compared with LiOH/Na2MoO4 inhibitor, the left shift range of polarization curves caused by PMA/SbBr3 inhibitor is wider. Both anodic and cathodic electrochemical reaction processes of copper may be inhibited by PMA/SbBr3 additive. It is reported that the electrode reactions of metals in LiBr solution are as follows[9]:
Anode:
M+nH2O→Mz+·nH2O+ze
Mz+·nH2O→M(OH)z+zH++(n-z)H2O
Cathode:
2H2O+2e→2OH-+H2
Thus PMA/SbBr3 inhibitor may prevent copper from dissolving in 55% LiBr solution and inhibit hydrogen evolution.
Polarization curves of copper and copper-nickel alloy in boiling 55%LiBr+ PMA/SbBr3 solution are exhibited in Fig.3. Compared with copper, both anodic and cathodic polarization curves of copper-nickel alloy shift left. The anodic polarization curve of copper-nickel alloy shows an obvious passive region near -300mV, and the width of it is about 150mV. Whereas the polarization curve of copper shows that copper keeps an active dissolution state in the same situation. With the potentials moving towards positive direction, its anodic polarization current density increases.
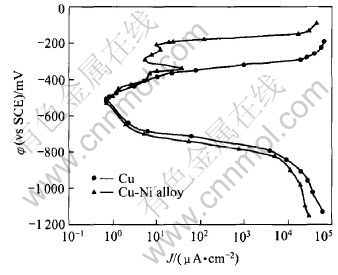
Fig.3 Polarization curves of copper and copper-nickel alloy in boiling 55%LiBr+PMA/SbBr3 solution
Curves in Fig.4 present the corrosion rates of copper and copper-nickel alloy in boiling 55%LiBr+PMA/SbBr3 solution. The corrosion rates of copper and copper-nickel alloy increase with the rise of solution temperature. At the same temperature, the corrosion rate of copper-nickel alloy is lower than that of copper, which indicates that the anti-corrosion performance of copper-nickel alloy is better than that of copper. This result coincides with the results displayed in Fig.3. According to Refs.[5, 10], with the increase of nickel content in copper-nickel alloy, impedance of alloy increases and corrosion potential moves towards positive direction in LiBr solution. It is because that adding nickel into copper can improve the thermodynamic stability of metal. It is the main factor that causes the different corrosion behaviors of copper and copper-nickel alloy in LiBr solution. Corrosion rates of copper and copper-nickel alloy in boiling 55%LiBr solution are 114.35μm/a and 96.29μm/a, respectively. When PMA/SbBr3 inhibitor is added into 55%LiBr solution, their corrosion rates reduce to 67.48μm/a and 38.14μm/a, respectively. It is obvious that the corrosion of copper and copper-nickel alloy may be inhibited by this additive to a great extent in 55%LiBr solution.
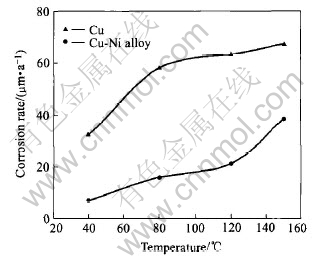
Fig.4 Effects of temperature on corrosion rate of copper and copper-nickel alloy in 55%LiBr+PMA/SbBr3 solution
As observed from the EPMA surface microphotograph of copper immersed in boiling 55%LiBr+PMA/SbBr3 solution for 200h(Fig.5), copper surface displays a general corrosion state and some protective films exist on it. Proved by Fig.5(b) and Fig.5(c), the surface layer contains elements Sb, O and Br. This indicates that Sb and O take part in the process of the films forming. PMA/SbBr3 inhibitor is a mixture of complex transitional metal anion and compound SbBr3. PMA is a heteropoly complex anion and its chemical formula is [PMo12O40]3-. PMA has a high solubility in LiBr solution, which may be helpful to maintain an effective concentration in solution. It has the effect of inhibiting hydrogen evolution. The chemical valence of element Mo in anion [PMo12O40]3- is +6, so PMA shows a strong oxidizing ability. Sb3+ also behaves an oxidizing ability. It may exist in LiBr solutions stably with PMA[11-14]. A protective layer comprising CuO, Cu2O and Sb may form on copper surface by PMA/SbBr3 inhibitor[15]. Electrochemical reactions of copper in 55% LiBr+PMA/SbBr3 solution are shown as follows:
Anode:
Cu+2OH-→CuO+H2O+2e(1)
2Cu+2OH-→Cu2O+H2O+2e(2)
Cathode[14, 16]:
[PMo(Ⅵ)12O40]3-+2H2O+ne→
2OH-+[H2PMo(Ⅵ)12-nMo(Ⅴ)nO40](1+n) -(3)
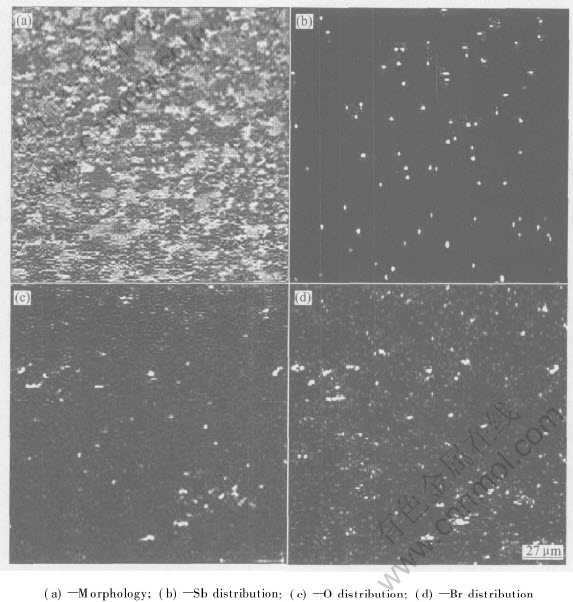
Fig.5 EPMA micrographs of copper immersed in boiling 55%LiBr+PMA/SbBr3 for 200h
Sb3++3e→Sb(4)
Following cathodic reaction may occur too:
2H2O+2e→2OH-+H2(5)
For the reactions above, corrosion rates of copper and copper-nickel alloy may be reduced by this film in LiBr solution. Cathodic reaction(3) can produce OH- so that the occurrence of cathodic reaction(5) will be inhibited. This proves that PMA has the effect of inhibiting hydrogen evolution.
Since [H2PMo(Ⅵ)12-nMo(Ⅴ)nO40](1+n)-, the product of cathodic reaction, can dissolve into LiBr solution easily and its concentration is low, element Mo cannot be detected on the metal surface. It is found that elements Sb and O are absorbed on copper surface(Fig.5). This evidence proves that the discussion of inhibition mechanism above is reasonable. Therefore, because of the synergistic effect of PMA and Sb3+, the protective layer comprising CuO, Cu2O and Sb will be formed on the surface of copper and copper-nickel alloy, which can prevent Br- from diffusing to the surface of metals. As a result, the anticorrosion performance of copper and copper-nickel alloy may be improved by PMA/SbBr3 inhibitor in 55% LiBr solution.
4 CONCLUSIONS
1) In boiling 55% LiBr+ PMA/SbBr3 solution, corrosion rates of copper and copper-nickel alloy are 67.48μm/a and 38.14μm/a, respectively. Corrosion of copper and copper-nickel alloy may be inhibited by PMA/SbBr3 inhibitor to a great extent in 55% LiBr solution.
2) PMA/SbBr3 inhibitor can inhibit both anodic and cathodic electrochemical reactions of copper and copper-nickel alloy in 55%LiBr solution, so it belongs to complex inhibitor.
3)The corrosion inhibition mechanism of PMA/SbBr3 in 55%LiBr solution is suggested as follows: PMA has the effect of inhibiting hydrogen evolution. For [PMo12O40]3-, the anion of PMA, has a strong oxidizing effect, Sb3+ also behaves an oxidizing effect. Sb3+ may exist in LiBr solutions stably with PMA. Because of the synergistic effect of PMA and Sb3+, the protective film comprising CuO, Cu2O and Sb formed on copper and copper-nickel alloy surface may prevent Br- from diffusing to the surface of metals. As a result, the anticorrosion performance of copper and copper-nickel alloy may be improved by PMA/SbBr3 inhibitor in 55% LiBr solution.
REFERENCES
[1]Jiangzhou S, Wang R Z. Experimental research on characteristics of corrosion-resisting nickel alloy tube used in triple-effect LiBr/H2O absorption chiller [J]. Applied Thermal Engineering, 2001, 21(11): 1161-1173.
[2]Ziegler F. Recent developments and future prospects of sorption heat pump systems [J]. International Journal of Thermal Science, 1999, 38(3): 191-208.
[3]Ziegler F. State of the art in sorption heat pumping and cooling technologies [J]. International Journal of Refrigeration, 2002, 25(4): 450- 459.
[4]LIANG Cheng-hao, AN Xiao-xia. Study on the application of Li2CrO4 in LiBr solution [J]. Refrigeration, 2000, 19(2): 23-26.(in Chinese)
[5]HUANG Nai-bao, LIANG Cheng-hao, TONG Da-wei. Effect of inhibitors on corrosion behavior of copper-nickel in concentrated lithium bromide solution at high temperature [J]. Trans Nonferrous Met Soc China, 2002, 12(3): 424-428.
[6]GUO Jian-wei, LIANG Cheng-hao. Corrosive behavior of carbon steel in high temperature and high concentration lithium bromide solution [J]. Journal of Dalian University of Technology, 2000, 40(5): 554-556.(in Chinese)
[7]HUANG Nai-bao, LIANG Cheng-hao. Corrosion behavior of carbon steel in lithium bromide solution [J]. Journal of the Chinese Chemical Society, 2003, 50(5): 979-984.
[8]HAN Ke-ping, FANG Jing-li. A protective coating of P-Mo-V heteropoly acid on steel [J]. Journal of Electron Spectroscopy and Related Phenomena, 1997, 83(1): 93-98.
[9]Tanno K, Itoh M, Takahashi T, et al. The corrosion of carbon steel in lithium bromide solution at moderate temperatures [J]. Corrosion Science, 1993, 34(9): 1441-1451.
[10]HUANG Nai-bao, LIANG Cheng-hao. The development of corrosive research of copper and copper alloy in lithium bromide absorption chiller [J]. Refrigeration, 2001, 20(2): 25-28.(in Chinese)
[11]Verma, Shyam Kumar, Mekhjian, et al. Corrosion Inhibiting Solutions and Processes for Refrigeration Systems Comprising Halides of a Group Va Metallic Element [P]. US 6033595, 2000.
[12]Verma, Shyam Kumar, Mekhjian, et al. Corrosion Inhibiting Solutions and Processes for Refrigeration Systems Comprising Halides of a Group Va Metallic Element [P]. US 6267908, 2001.
[13]Verma, Shyam Kumar, Mekhjian, et al. Corrosion Inhibiting Solutions for Refrigeration Systems Comprising Heteropoly Complex Anions of Transition Metal Elements [P]. US 6004475, 1999.
[14]Verma, Shyam Kumar, Mekhjian, et al. Corrosion Inhibiting Solutions and Processes for Refrigeration Systems Comprising Heteropoly Complex Anions of Transition Metal Elements Additional Additives [P]. US 6004476, 1999.
[15]HUANG Nai-bao, LIANG Cheng-hao. Research on corrosion resistance of copper at high temperature and in high concentration LiBr solution [J]. Journal of Dalian University of Technology, 2001, 41(6): 667-670.(in Chinese)
[16]WANG Rui, BAO Xiao-jun. Reaction mechanism of hydrogen sulfide with sodium phosphomolybdate [J]. Acta Petrolei Sinica(Petroleum Processing Section), 2000, 16(6): 70-73.(in Chinese).
(Edited by YANG Bing)
Received date: 2004-07-20; Accepted date: 2005-03-22
Correspondence: LIANG Cheng-hao, Professor; Tel: +86-411-88993926; E-mail: liangch@chem.dlut.edu.cn


