
Wear property of high-resistivity carbon brushes made with and without MoS2 in variable humidity
HU Zhong-liang(胡忠良), CHEN Zhen-hua(陈振华),
XIA Jin-tong(夏金童), DING Guo-yun(丁国芸)
College of Materials Science and Engineering, Hunan University, Changsha 410082, China
Received 17 April 2007; accepted 23 August 2007
Abstract:
Four kinds of high-resistivity carbon brushes with MoS2 contents of 0%, 2%, 4% and 6% (mass fraction) were prepared, respectively. Wear tests of brushes were conducted on an alternate current(AC) motor. Scanning electron microscopy(SEM) and energy dispersive X-ray analysis(EDS) were used to analyze the worn surface of brushes, and a thermocouple was used to measure the bulk temperature of the brush. The results show that wear rate of brush made without MoS2 in 10% relative humidity(RH) is two times larger than that in 50% RH, whereas the wear rates change little for the brushes made with MoS2. The wear of brushes has much to do with the surface film. In low humidity, the surface film can not be formed for the former brush while a sulfur layer can be formed for the latter brushes,which can reduce sparks, frictional heat and wear rate, and play a role like the water film in high humidity.
Key words:
molybdenum disulfide; wear; high-resistivity brushes; humidity;
1 Introduction
In electrical-rotor systems, the purpose of electric brushes is to conduct current from the rotating part to the stationary part of a motor, and the performances of brushes are of vital importance for motors to run normally. Many scientists have been studied to develop the brush materials with high performances[1-4]. Recently, high speed motors have been largely developed to satisfy the household appliance industry, accordingly, high-resistivity carbon brushes have been invented to match the motors[5-6], and they are often made of graphite, resin binder and solid lubricant. At present considerable efforts have been made to study this type of brushes, however, these investigations were mainly focused on the effects of formula and manufacturing process parameters on the properties of brushes[7], and related research on the lubricant for the brushes has rarely been found.
Lubricants play a key role for the wear of brushes [8], and molybdenum disulfide is an excellent solid lubricant for brush materials, especially under extreme conditions such as low humidity while graphite loses its lubrication, so it is often chosen as the lubricant of brushes. Although some studies on MoS2 have been performed, most investigations are on MoS2 matrix coating and metal matrix composites[9-11]. In high-resistivity brushes, what role does MoS2 play as a lubricant and how does it influence the wear of brushes in electrical engineering have rarely studied systematically.
This work is original and different from most investigations on the wear of brushes in our test method. Most experiments were conducted on a pin-on-disc machine. The results may not be applied to AC motors, because in AC motor, the change of current flow in commutated windings leads to the dissipation of electromagnetic energy that is stored in windings mainly in the intercontact space. This phenomenon must influence the wear of brushes and the lubricating effect of MoS2. The wear tests were performed on a high speed AC motor in our study, and the data was convinced and in accordance with the actual situation. It is expected that the study will be meaningful and have theoretical guide to develop the high-resistivity carbon brushes.
2 Experimental
2.1 Sample preparation and characterization
The manufacturing process of high-resistivity carbon brushes is shown in Fig.1.
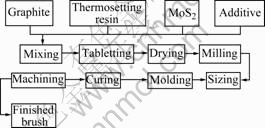
Fig.1 Manufacturing process for carbon brushes
In first step, all materials were mixed for 1 h, the mixture was then tabletted, dried, milled and sized by a 74 μm sieve.
In second step, the blend was molded to cuboids at 100 MPa.
In third step, the molded specimens were cured at 180 ℃ for 3 h. Finally, the specimens were machined to the desired shape of 5.2 mm×5.5 mm×32.0 mm.
All carbon brushes contained the same relative content of materials except MoS2, which were 75% graphite (size less than 74 μm), 22% modified phenolic resin and 3% additive (mass fraction). Four kinds of high-resistivity carbon brushes with contents of 0%, 2%, 4%, 6% MoS2 (mass fraction. %) were prepared, which were hereinafter designated as Brush A, B, C and D, respectively.
2.2 Wear rate tests and characterization for brushes
Wear tests of brushes were performed on a low power AC motor (type RB5540M220A). Its commutator with 12 bars was made of copper with diameter of 17.0 mm. All tests were carried out when the motor was in free load and its rotation speed was 14 860 r/min. The schematic diagram of the test AC motor is shown in Fig.2.
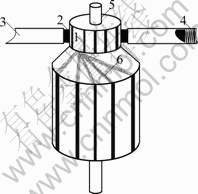
Fig.2 Schematic diagram of test motor: 1 Commutator bar; 2 Brush; 3 Brush holder; 4 Contact force spring; 5 Bearing; 6 Winding
A humidifier was used to control the surrounding humidity.
The wear tests of brushes were measured until the sliding time reached 20 h and the commutator was polished with a very fine Al2O3 paper before each experiment. To measure the wear volume, the mass loss of brushes was obtained with a balance of 0.1 mg precision, and then the value was divided by the bulk density of brushes. All the experiments were conducted
at 25 ℃ under 3 N/cm2 normal load.
The temperature of brushes was measured by a WRE-206S thermocouple with precision of 0.5 ℃, and the place where the temperature was measured was 3 mm from worn surface. The temperature was measured 3 times and average value was obtained as the bulk temperature.
The worn surface of the test brush was observed by SEM without cleaning so that all original features could be observed and the compositions of the surface film on the brush were analyzed by EDS.
3 Results and discussion
Fig.3 shows the wear volume variation of Brush A with the wear time in 10% relative humidity(RH) and 50% RH. From Fig.3, it can be seen easily that the slope of the curve for 50% RH is large at first, but after 5 h it decreases and almost keeps constant; while in 10% RH, the wear volume is nearly directly proportional to the wear time, and the value of slope is constant and larger than that in 50% RH.
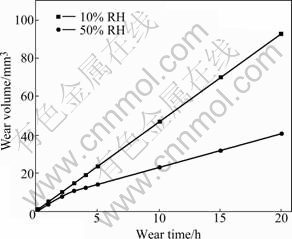
Fig.3 Wear volume variation of Brush A with wear time in different humidities
It is well known that the surface film is formed when a motor runs and it can act as lubricant and reduce the wear of brushes[12-13]. The worn surface micrographs of Brush A sliding after 1 h and 5 h in 50% RH are shown in Fig.4(a) and Fig.4(b), respectively.

Fig.4 Micrographs of worn surfaces of Brush A in 50% RH sliding after different time: (a) 1 h; (b) 5 h
As demonstrated by Fig.4, sliding after 1 h, the surface film is not well developed. It is thin and noncontinuous. But after 5 h, it is continuous and uniform and almost covers the whole surface of brushes. The main reason is that the rate of formation surface film is equal to that of damaging of surface film and it is nearly in a dynamic equilibrium and steady state after 5 h sliding. Above analysis is supported by the observation for the worn surfaces of commutator and brush that are covered by a uniform and stable film after 5 h sliding. So in 50% RH sliding after 5 h the wear of brushes becomes little and steady, whereas at initial stage it is large.
From Fig.3 one can also find that in 10% RH the wear rate approximately keeps unchanged from initial time and it is almost two times larger than that in 50% RH. It may be because the surface film can not be formed under such conditions, and this has been proved by SEM morphology of the worn surface in 10% RH shown in Fig.5. From the figure one can clearly see that there are fragmentary grains and dust on the brush surface and the surface film can not be found. To further investigate the brush surface, EDS was adopted to analyze the worn surface in different humidities and the results are listed in Table 1, in which the data are presented by mole fraction. This shows that there is no copper element in 10% RH while in 50% RH copper content reaches 4.1 %.
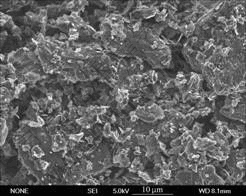
Fig.5 Morphology of worn surface of Brush A in 10% RH
Table 1 Surface element analysis of Brush A in 50% RH and 10% RH
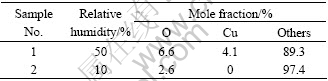
According to Ref.[14], copper in the surface film is in form of Cu2O, which can segregate copper and graphite grains, so the formation of Cu2O can reduce the wear of brushes. EDS data in Table 1 indicate that Cu2O film can not be formed in 10% RH, which leads to a high wear rate. The reason is that water vapour facilitates the formation of cuprous oxide protective layer and water might act as a catalyst to form cuprous oxide. Reactions on the interface may be expressed as
H2O=H++OH- (1)
O2+4H++Cu=Cu2O+2H2O (2)
Water can be dissociated into H+ and OH– ion under current, and standard electrode potential of O2/H+ is 1.229 V, which increases oxidizing ability of O2 and can oxidize Cu into Cu2O. In the absence of water or with little quantity of water, oxidizing ability of O2 is not strong enough to oxidize Cu into Cu2O.
On the other hand, based on the theories of Savage and Lancaster[15-17], in high humidity, the number of water molecules condensation is more than that of evaporation until a transient water monolayer is formed on the surface of brushes, thus water vapor molecules tend to cover the whole exposed graphite surface. So the free surface energy is lowered, which reduces cohesion and adhesion between contact areas, this making friction and wear decrease.
In low humidity, graphite cannot obtain its saturated surface for the low water pressure, the adsorption water film only covers a part of graphite surface, the free surface energy is high and the contact molecules between graphite and copper may seize, which it makes the friction and the wear of brushes increase largely. The fragmentary grains shown in Fig.5 are caused by the cohesion and adhesion wear of brushes.
As far as Brushes B, C and D are concerned, the wear behaviour is completely different from Brush A. Fig.6 demonstrates the relationship between the wear volume and time for Brush C, which represents a typical curve for brushes made with MoS2. Compared with Fig.3, there exist two significant differences in Fig.6. First, the wear rates of these brushes change little in different humidity. Second, in different humidity, a similar rule is suitable for wear rate of brushes, that is, when the tests begin the wear rate is large, but after a period of time it becomes small and almost constant.
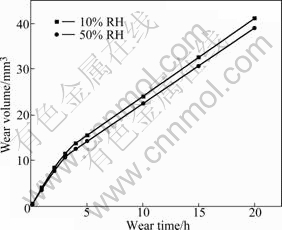
Fig.6 Wear volume evolution of Brush C with wear time in different humidities
The wear rates of brushes in 50% RH and 10% RH are listed in Table 2, and they were measured after 5 h sliding. From Table 2 it is found that the wear rate decreases with increasing MoS2 content, yet the difference is not large. Humidity has far less influence on Brushes B, C and D than Brush A, obviously this is due to the effect of MoS2. To further investigate the effect of MoS2, the SEM morphologies of the worn surfaces of Brush C in different humidities are presented in Fig.7. There are generally smooth and uniform surface films on the worn surfaces of Brush C, and they act as lubricant and reduce the wear of brushes. Although there exist some differences between these two worn surfaces, which may be caused by different compositions of them, it has little influence on the wear of brushes.
Table 2 Wear rates of brushes in different humidity (mm3/h)

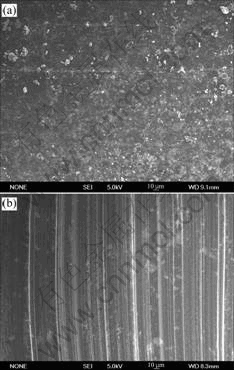
Fig.7 Worn surfaces of Brush C in different humidities: a) 50% RH; (b) 10% RH
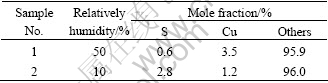
EDS results are listed in Table 3, and it can be clearly seen that sulfur content increases several times while copper content decreases largely. Because in low humidity, the water film cannot be formed in absent of water, here sulfur in the surface maybe act as lubricant. The reaction may be expressed as
2MoS2+3O2=4S+2Mo2O3 (1)
Table 3 Surface element analysis of Brush C in 50% RH and 10% RH
JOHNSON and VAUGHN[18] proved that an adsorbed layer of amorphous sulfur is generated during the sliding process. Amorphous sulfur is adsorbed on the worn surface of brushes, and segregates brush and commutators. It can lower the surface energy and play a role like water film in high humidity. Thus brushes made with MoS2 keep lubrication in 10% RH.
On the other hand, different mechanisms may exist in 50% RH and in 10% RH, which can explain the difference between Fig.7(a) and Fig.7(b). In 50% RH graphite dominates the lubrication effect, while in 10% RH MoS2 plays a key role of lubrication, and MoS2 has little influence on the wear of brushes in high humidity.
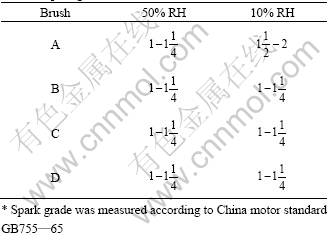
According HOLM’s theory on electric contact, the wear of brushes is composed of mechanical wear, electrical wear and spark wear[19]. Under the experimental condition, the rotating speed of motor is very high and sparks are often induced, which does much harm to the wear of brushes. Spark grade of brushes in different humidities is listed in Table 4. It is found that in 50% RH the spark grade is almost same for all brushes. But in 10% RH sparks for Brush A become much larger while Brushes B, C and D have almost same large sparks in 50% RH.
In 10% RH, the lubricating film cannot be formed on the surface of Brush A, and the direct contact between graphite and copper could induce short-circuiting sparks easily. But for brushes containing MoS2, sulfur film, Cu2O and MoS2 can restrain sparks efficiently due to their insulating property, so sparks change little.
Table 4 Spark grade of brushes in different humidities
The bulk temperature of brushes was measured and the results are listed in Table 5. It is thought that the bulk temperature of brushes has much influence on the wear of carbon brushes[20]. The binder can occur oxidation reaction and the bonding strength between brush materials can be weakened under a high temperature and also it makes the surface film become rough and easily worn off.
Table 5 Bulk temperature of brushes in different humidities (℃)

The data in Table 5 show some similarity to that in Table 4. That is, in different humidities, the temperature changes much for Brush A while only a little temperature rise happens for other brushes made with MoS2. In 10% RH a lubricating film cannot be formed on the surface of Brush A, graphite grains and copper may seize and it will increase the friction coefficient and thus much more frictional heat is produced, so a high temperature is obtained on the surface of Brush A in 10% RH. An opposite situation is applied to brushes made with MoS2, in 10% RH a sulfur film is formed on the surface of brushes, and frictional heat does not change much, so the temperature changes little. In general, the high temperature of Brush A in 10% RH also aggravates the wear of brushes.
4 Conclusions
1) For high-resistivity carbon brushes made without MoS2, the wear rate in 10% RH is about 2 times greater than that in 50% RH; correspondingly, for brushes made with MoS2. The wear rate changes little.
2) Surface film is of importance to the wear of brushes, the wear of brushes is low when a smooth surface film is formed, otherwise the wear is large.
3) MoS2 can help to form a sulfur layer on the brush surface, which can act as lubricant, so the wear rate is low in low humidity.
4) In low relative humidity, sulfur film can also restrain sparks and keep motor run normally, reduce frictional heat and induce a relatively low bulk temperature of brushes. All these factors help to reduce the wear of brushes.
References
[1] GAO Qiang, WU Yu-ying, ZHANG Guo-ding, HONG Qin, XIAO Xue-ming. Effect of carbon fiber on property of copper-graphite composite materials [J]. The Chinese Journal of Nonferrous Metals, 2000, 10: 97-101. (in Chinese)
[2] UECKER A. Lead-free carbon brushes for automotive starters [J]. Wear, 2003, 255: 1286-1290.
[3] FENG Yi, YUAN Hai-long, ZHANG Min. Processing and electrical conductivity of carbon nanotubes-silver composites [J]. The Chinese Journal of Nonferrous Metals, 2004, 14: 1451-1455. (in Chinese)
[4] ZHANG Lei, ZHOU Ke-chao, LIU Wen-sheng, ZHOU Rong-xing, LI Shi-wei. Preparation and properties of Ag-Cu-MoS2 brushes materials [J]. The Chinese Journal of Nonferrous Metals, 2005, 15: 1766-1769. (in Chinese)
[5] XIA Jin-tong, LU Xue-feng, LI Yan, SHAO Hao-ming. Study on the preparation process and properties of high-resistance carbon brush [J]. Journal of Hunan University (Natural Science), 2005, 32: 88-93.
[6] ROBERGE R. Carbon brush performance and application in the pulp and paper environment [J]. National Electrical Products, 2001, 6: 184-191.
[7] LIU Yun, WU Yu-ying, HONG Qin, XIAO Xue-ming. Properties of electric friction materials with resins [J]. Materials for Mechanical Engineering, 2002, 11: 42-45.
[8] LI Xiu-bing, GAO Yi-ming, XING Jian-dong, WANG Yu, FANG Liang. Wear reduction mechanism of graphite and MoS2 in epoxy composites [J]. Wear, 2004, 257: 279-283.
[9] XIONG Dang-sheng, PENG Chao-qun, HUANG Qi-zhong. Development of MoS2-containing Ni-Cr based alloys and their high-temperature tribological properties [J]. Trans Nonferrous Met Soc China, 1998, 8(2): 226-229.
[10] XIONG Dang-sheng, PENG Chao-qun, LIU Jin-long. Effects of MoS2 on mechanical and tribological properties of NiCr-based alloys [J]. Trans Nonferrous Met Soc China, 2000, 10: 328-331.
[11] KATO H, TAKAMA M, IWAI Y, WASHIDA K, SASAKI Y. Wear and mechanical properties of sintered-tin composites containing graphite or molybdenum disulfide [J]. Wear, 2003, 255: 573-578.
[12] DIES K. Die reiboxidations als chemische mechaniche Vergang [M]. Forschungsberichte: Techn Mitteilungen Gruppe, 1942: 5127.
[13] HU Zhong-liang, CHEN Zhen-hua, XIA Jin-tong. Study on surface film in the wear of electrographite brushes against copper commutators for variable current and humidity [J]. Wear, 2008, 264: 10-17.
[14] SPRY W J, SCHERER P M. Copper oxide film formation at a sliding carbon–copper interface [J]. Wear, 1961, 4: 137-149.
[15] SAVAGE R H. Carbon brush contact films [J]. Gen Elec Rev, 1945, 48: 13-20.
[16] SAVAGE R H. Graphite lubrication [J]. J Appl Phys, 1948, 19: 1-10.
[17] LANCASTER J K, PRITCHARD J R. On the ‘dusting’ wear regime of graphite sliding against carbon [J]. J Phys D: Appl Phys, 1980, 13: 1551-1564.
[18] JOHNSON V R, VAUGHN G W. Investigation of the mechanism of MoS2 lubrication in vacuum [J]. J Appl Phys, 1956, 27: 1173-1179.
[19] HOLM R. Electric contacts [M]. Berlin: Springer Verlag, 1958.
[20] KONCHITS V V, KIM C K. Electric current passage and interface heating [J]. Wear, 1999, 232: 31-40.
Foundation item: Project(59972009) supported by the National Natural Science Foundation of China
Corresponding author: HU Zhong-liang; Tel: +86-731-8821564; E-mail: david10103@sina.com
[2] UECKER A. Lead-free carbon brushes for automotive starters [J]. Wear, 2003, 255: 1286-1290.
[15] SAVAGE R H. Carbon brush contact films [J]. Gen Elec Rev, 1945, 48: 13-20.
[16] SAVAGE R H. Graphite lubrication [J]. J Appl Phys, 1948, 19: 1-10.
[19] HOLM R. Electric contacts [M]. Berlin: Springer Verlag, 1958.


