文章编号:1004-0609(2008)S1-0290-06
钛掺杂LiFePO4的还原插锂合成及其性能
伍 凌,王志兴,李新海,李灵均,郑俊超,郭华军,彭文杰
(中南大学 冶金科学与工程学院, 长沙 410083)
摘 要:
用共沉淀法制备掺钛前驱体FePO4·2H2O,对FePO4·2H2O经常温还原插锂合成LiFePO4的前驱混合物,后经热处理得橄榄石型LiFePO4;用SEM,XRD和恒流充放电等对样品进行表征,考察Ti掺杂和合成温度对LiFePO4的物理和电化学性能的影响。研究结果表明,在600 ℃时合成的Ti掺杂样品具有优异的电化学性能,该样品在0.1C,1C和2C倍率下的首次放电比容量分别为150,130和125 mA?h/g,循环40次后的放电比容量均无衰减。
关键词:
中图分类号:TM 912.9 文献标识码:A
Reduction and lithiation synthesis and properties of Ti-doped LiFePO4
WU Ling, WANG Zhi-xing, LI Xin-hai, LI Ling-jun, ZHENG Jun-chao, GUO Hua-jun, PENG Wen-jie
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Ti-doped precursors (FePO4·2H2O) were prepared by co-precipitation method. Olivine-type LiFePO4 were synthesized by sintering the LiFePO4 precursor-mixtures which were obtained via reduction and lithiation of FePO4·2H2O at room temperature. The samples were characterized by scanning electron microscopy, X-ray diffractometry and electrochemical charge and discharge tests. Effects of Ti-doping and sintering temperature on physical and electrochemical performance of LiFePO4 were investigated. The results show that the Ti-doped sample sintered at 600 ℃ has excellent electrochemical performance. Its initial discharge capacities are 150, 130 and 125 mA?h/g at 0.1C, 1C and 2C rates, respectively, and no capacity fading is found after 40 cycles.
Key words: lithium ion battery; LiFePO4; co-precipitation; room temperature reduction; Ti-doping
橄榄石结构的LiFePO4因其具有理论比容量高(170 mA?h/g),电压适中(3.4 V),循环性能和安全性能好,无毒,价廉等优点,成为新一代锂离子电池正极材料的有力竞争者。然而,其具有极低的导电率(10-9~ 10-10 S/cm)[1]和锂离子扩散速率(1.8×10-14 cm2/s)[2],阻碍了其大规模应用,因此,如何提高LiFePO4的导电性成为当前研究热点。目前,主要通过高价金属阳离子掺杂[1]、制备LiFePO4/金属粉末[3]或LiFePO4/C[4]复合材料以及优化合成工艺[5]来提高LiFePO4的导电性。对LiFePO4掺杂的方法大多为固相法[1, 6]和溶胶-凝胶法[7-8],而采用共沉淀法的报导较少。在此,本文作者采用一种新的掺杂方式,即首先通过共沉淀得到钛元素均匀掺杂的前驱体FePO4·2H2O,然后,通过还原插锂合成LiFePO4。在还原插锂工序中,选用一种新型的还原剂——乙二酸,该还原剂在常温下与铁源及锂源球磨混合得到浅绿色的无定形LiFePO4前驱混合物,后经热处理得橄榄石型LiFePO4。关于常温还原的研究见文献[9]。在此,本文作者重点研究Ti掺杂和合成温度对LiFePO4的结构、形貌以及电化学性能的影响。
1 实验
1.1.1 前驱体的制备
分别称取1 mol FeSO4·7H2O(AR,广东省台山市化工厂),1 mol H3PO4(AR,汕头市西陇化工厂),x mol(x=0,0.03) Ti(SO4)2·H2O(CP,国药集团化学试剂有限公司),溶于去离子水中,在强烈搅拌下加入足量H2O2(AR,汕头市西陇化工厂),用NH3·H2O(AR,汕头市西陇化工厂)调节pH值至2.1左右,反应10 min,将得到的乳白色沉淀洗涤、过滤数次,然后,于120 ℃干燥12 h即得前驱体FePO4·2H2O。
1.1.2 LiFePO4的制备
按化学计量比称取一定量的前驱体、Li2CO3(AR,新余市赣丰锂业有限工司)和乙二酸(AR,国药集团化学试剂有限公司),以乙醇为介质,在室温下球磨3 h后得到浅绿色的LiFePO4前驱混合物,将混合物于80 ℃烘干后置入程序控温管式炉,在高纯氩(99.999%)气氛中于不同温度(550,600和650 ℃)煅烧12 h,随炉冷却即得橄榄石型LiFePO4。将600 ℃时合成的掺杂和未掺杂样品分别记为Doping和Undoping。
本实验采用日本Rigaku D/max2550VB+18 kW转靶X射线衍射仪进行物相分析,分析条件为:Cu Kα辐射,40 kV,300 mA,步宽0.02?。用WinPLOTR软件计算晶胞常数,用Scherrer公式计算晶粒尺寸。采用JEOL公司的JSM-5600LV扫描电镜在20 kV下观察样品的表面形貌。用电感耦合光谱ICP离子探针(IRIS intrepid XSP等离子体发射光谱仪,Thermo electron corporation)测定前驱体的钛含量;用重铬酸钾滴定法(SnCl2-HgCl2测铁法)测定前驱体的铁含量。
将LiFePO4、导电碳黑和粘结剂(PVDF)按质量比8?1?1混合,以铝箔为基体制备成直径为14 mm的正极片,将正极片与负极片(锂)、电解液(浓度为1 mol/L的LiPF6/EC+EMC+DMC,体积比为1?1?1)和隔膜(Celgard 2300 PP/PE/PP)在充满氩气的手套箱中组装成CR2025型扣式电池,电池静置12 h后用新威BTS- 5 V/1 mA电池测试系统进行测试,测试条件为:室温,2.5~4.1 V,以0.1C,1C和2C恒流充放电。交流阻抗测试在美国CHI660电化学工作站上完成,测量频率为0.01 Hz至100 kHz,正弦波振幅为5 mV。
2 结果与讨论
掺Ti前驱体(FePO4·2H2O)的Ti与Fe摩尔比为2.97%,与目标值(3%)非常接近,表明共沉淀制备前驱体时Ti几乎全部进入前驱体中。
图1所示为掺杂与未掺杂LiFePO4的XRD谱,与LiFePO4的标准谱(JCPDS No. 40-1499)相比,两样品均为有序的橄榄石结构,且均未发现有杂相,说明少量的Ti4+掺杂不会改变LiFePO4的晶体结构。Ti4+可能占据M1(Li)位或者M2(Fe)位,当占据M1位时,Ti4+与LiFePO4形成固溶体,不会产生杂相[7, 10];而当Ti4+占据M2位时可能出现Li3PO4,Fe3P和Li3Fe2(PO4)3等杂相[8, 11]。此外,根据CHUNG等[1]提出的掺杂机理,Ti4+更趋向于占据M1(Li)位。因此,断定Ti4+占据Li位与LiFePO4形成了固溶体。
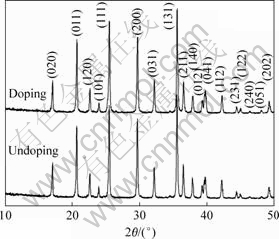
图1 掺杂与未掺杂LiFePO4的XRD谱
Fig.1 XRD patterns of doped and undoped LiFePO4
表1所列为掺杂与未掺杂样品的晶胞参数和晶粒粒径。可见,与未掺杂样品相比,掺杂样品的晶胞参数a,b,c和晶胞体积均稍减小,这是由于:
表 1 掺杂与未掺杂LiFePO4的晶胞参数和晶粒粒径
Table 1 Lattice parameters and crystallite size of doped and undoped LiFePO4
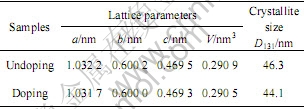
1) Ti4+的有效离子半径(0.0605 nm)比Li+的有效离子半径(0.0760 nm)小[12],Ti4+占据部分M1位后引起晶胞收缩。
2) 1 mol Ti4+占据M1位产生4 mol Li缺陷,导致Li+的浓度降低,从而引起晶胞收缩。
Li缺陷的产生使得LiFePO4中形成一定量的Fe3+/Fe2+混合价态,有助于提高LiFePO4导电性[1, 13]。
另外,掺杂样品的晶粒粒径(D131)比未掺杂样品的小,这说明Ti4+能抑制LiFePO4晶粒长大。
图2所示为不同温度下合成的Ti掺杂LiFePO4的XRD谱。随着合成温度的升高,LiFePO4衍射峰的相对强度逐渐增强,由此可见,升高合成温度有利于提高LiFePO4的结晶度。然而,合成温度的提高也使得LiFePO4的晶粒粒径增大,在550,600和650 ℃合成样品的晶粒粒径(D131)分别为41.0,44.1和49.4 nm。
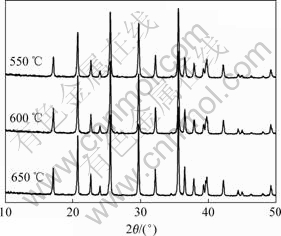
图2 不同温度下合成的Ti掺杂LiFePO4的XRD谱
Fig.2 XRD patterns of Ti-doped LiFePO4 synthesized at different sintering temperatures
图3所示为掺杂前后LiFePO4的SEM图。可见,未掺杂样品与掺杂样品相比,二者都同时存在细小的一次颗粒(粒径<1 μm)和由一次颗粒团聚而成的二次颗粒,但前者颗粒的团聚程度明显比后者严重得多,这说明Ti4+的掺入能有效地抑制颗粒团聚,使材料细化。颗粒的细化增加了材料的比表面积,有利于提高大电流放电时LiFePO4的利用率[14]。

图3 掺杂与未掺杂LiFePO4的SEM图
Fig.3 SEM images of doped and undoped LiFePO4: (a) Undoping; (b) Doping
图4所示为不同温度下合成的Ti掺杂LiFePO4的SEM图。可见,随着合成温度的升高,LiFePO4的颗粒团聚越来越严重。结合图2和图4可知,于650 ℃时合成的LiFePO4虽然结晶度高,但颗粒团聚太严重,使得Li+在LiFePO4中扩散的距离增大[15]。相对而言,于600 ℃时合成的LiFePO4颗粒细小且具有较高的结晶度。
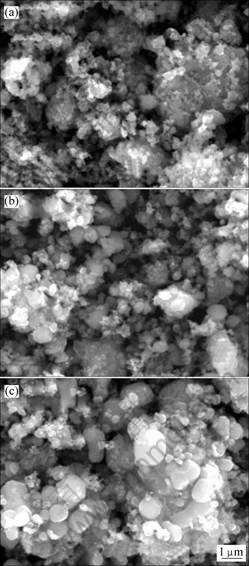
图4 不同温度下合成的Ti掺杂LiFePO4的SEM图
Fig.4 SEM images of Ti-doped LiFePO4 synthesized at different sintering temperatures: (a) 550 ℃; (b) 600 ℃; (c) 650 ℃
图5所示为掺杂与未掺杂LiFePO4样品的交流阻抗谱,图中2条谱线均由1个半圆和1条直线组成,高频区的半圆对应于电解质/氧化物电极界面的电荷传输阻抗(Zct),低频区的直线代表Li+在电极材料中扩散所引起的Warburg阻抗(Zw)[16]。由图 5可知,Ti的掺入使得Zct从830 Ω降低到130 Ω,这说明Ti掺杂能大幅度提高电解质/氧化物电极界面的电荷传递速率;另外,Ti的掺入也使得低频区直线的斜率增 大,即Warburg阻抗减小,从而有利于Li+在电极材料中的扩散。Zct和Zw减小有利于克服充放电过程中的动力学限制,使得Li+在LiFePO4颗粒中的脱/嵌深度提高,降低Li+在颗粒内部和表面的浓度差,从而有助于材料比容量的提高和循环性能的改善。
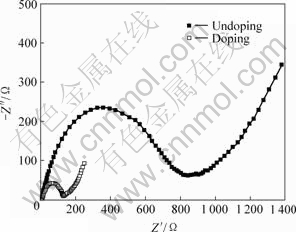
图5 掺杂与未掺杂LiFePO4的Nyquist图
Fig.5 Nyquist plots of doped and undoped LiFePO4
图6和图7所示分别为掺杂和未掺杂样品在不同倍率下的首次放电曲线和循环性能曲线。由图6可知,掺杂样品在0.1C,1C和2C倍率下的首次放电比容量分别为150,130和125 mA?h/g,比未掺杂样品(144,113和98 mA?h/g)分别提高4.2%,15.0% 和27.6%。由图7可知,2种样品在低倍率下均具有很好的循环性能,未掺杂样品和掺杂样品在0.1C倍率下循环20次后的放电比容量分别为144和151 mA·h/g,与首次容量相比均无衰减。然而,二者在大倍率下的循环性能相差很大,未掺杂样品在1C和2C倍率下循环40次后的放电比容量分别为104和66 mA?h/g,相对于首次容量分别衰减8.0%和32.7%;而掺杂样品在1C和2C倍率下循环40次后的放电比容量分别为133和130 mA?h/g,相对于首次容量不仅没有衰减,反而有所升高。根据前面的分析,Ti的掺入对LiFePO4电化学性能有显著改善的原因可归结如下:
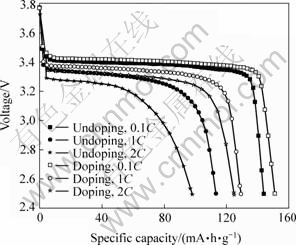
图6 掺杂与未掺杂LiFePO4在不同倍率下的首次放电曲线
Fig.6 Initial discharge curves of doped and undoped LiFePO4 at different rates
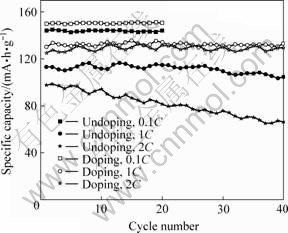
图7 掺杂与未掺杂LiFePO4在不同倍率下的循环性能
Fig.7 Cycling performance of doped and undoped LiFePO4 at different rates
1) Fe3+/Fe2+混合价态的形成有助于提高LiFePO4导电性;
2) Ti4+的掺入能有效地抑制颗粒团聚,材料的细化有利于提高大电流放电时LiFePO4的利用率;
3) 电荷传输阻抗Zct和Warburg阻抗Zw减小有利于克服充放电过程中的动力学限制,从而有助于材料比容量的提高和循环性能的改善。
图8所示为不同温度下合成的Ti掺杂LiFePO4在1C倍率下的首次充放电曲线和循环性能曲线。由图8可知,在550,600和650 ℃合成的样品在1C倍率下的首次放电比容量分别为107,130和121 mA·h/g,循环40次后的放电比容量分别为100(衰减6.5%),132 (升高1.5%)和116 mA·h/g(衰减4.1%)。从前面的分析可知,当合成温度太低(550 ℃)时,虽然LiFePO4的粒径细小,但结晶度较低,因此,放电容量不高,放电平台较短,循环性能较差;而当合成温度太高(650 ℃)时,虽然LiFePO4的结晶度较高,但颗粒团聚得较大,增大了Li+在LiFePO4中扩散的距离。600 ℃时合成的LiFePO4颗粒细小且具有较高的结晶度,因此,电化学性能优异。
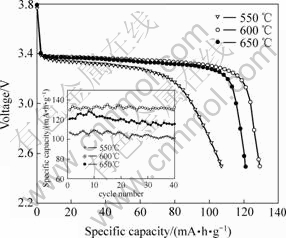
图8 不同温度下合成的Ti掺杂LiFePO4在1C倍率下的首次放电曲线和循环性能
Fig.8 Initial discharge curves at 1C rate and cycling performance of Ti-doped LiFePO4 synthesized at different sintering temperatures
3 结论
1) 采用共沉淀法将Ti掺入到前驱体FePO4·2H2O中,通过FePO4·2H2O对LiFePO4掺杂是一种新的掺杂途径;于常温还原插Li是一条合成LiFePO4的新思路。
2) Ti占据M1(Li)位与LiFePO4形成固溶体,不会影响其晶体结构,Ti的掺入可以有效地抑制LiFePO4颗粒的团聚。
3) 合成温度太低或太高都不利于LiFePO4电化学性能的改善。在600 ℃合成的Ti掺杂LiFePO4具有最优的电化学性能,该样品在大倍率下的放电性能良好,循环性能优异。
REFERENCES
[1] CHUNG S Y, BLOKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes[J]. Nature Materials, 2002, 1(2): 123-128.
[2] PROSINI P P, LISI M, ZANE D, PASQUALI M. Determination of the chemical diffusion coefficient of lithium in LiFePO4[J]. Solid State Ionics, 2002, 148: 45-51.
[3] CROCE F, EPIFANIO A D, HASSOUN J, DEPTULA A, OLCZAC T. A Novel concept for the synthesis of an improved LiFePO4 lithium battery cathode[J]. Electrochemical and Solid-State Letters, 2002, 5(3): A47-A50.
[4] CHEN Z, DAHN J R. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density[J]. Journal of the Electrochemical Society, 2002, 149(9): A1184-A1189.
[5] KIM D, KIM J. Synthesis of LiFePO4 nanoparticles in polyol medium and their electrochemical properties[J]. Electrochemical and Solid-State Letters, 2006, 9(9): A439-A442.
[6] WANG D, LI H, SHI S, HUANG X, CHEN L. Improving the rate performance of LiFePO4 by Fe-site doping[J]. Electrochimica Acta, 2005, 50: 2955-2958.
[7] HU Y, DOEFF M M, KOSTECKI R, FINONES R. Electrochemical performance of sol-gel synthesized LiFePO4 in lithium batteries[J]. Journal of the Electrochemical Society, 2004, 151(8): A1279-A1285.
[8] WANG G X, BEWLAY S, NEEDHAM S A, LIU H K, LIU R S, DROZD V A, LEE J F, CHEN J M. Synthesis and characterization of LiFePO4 and LiTi0.01Fe0.99PO4 cathode materials[J]. Journal of the Electrochemical Society, 2006, 153(1): A25-A31.
[9] ZHENG Jun-chao, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, ZHOU Shao-yun. LiFePO4 with enhanced performance synthesized by a novel synthetic route[J]. Journal of Power Sources, 2008, In Press.
[10] HU Guo-rong, GAO Xu-guang, PENG Zhong-dong, DU Ke, TAN Xian-yan, LIU Yan-jun. Influence of Ti4+ doping on electrochemical properties of LiFePO4/C cathode material for lithium-ion batteries[J]. Trans Nonferrous Met Soc China, 2007, 17: 296-300.
[11] SUN Yu-heng, LIU Xing-quan. Preparation and characterization of novel Ti-doped M-site deficient olivine LiFePO4[J]. Chinese Chemical Letters, 2006, 17(8): 1093-1096.
[12] 梁敬魁. 粉末衍射法测定晶体结构[M]. 北京: 科学出版社, 2003: 132-148, 776-848.
LIANG Jing-kui. Measurement of crystal structure by powder diffraction method[M]. Beijing: Science Press, 2003: 132-148, 776-848.
[13] THACKERAY M. Lithium-ion batteries: An unexpected conductor[J]. Nature Materials, 2002, 1(2): 81-82.
[14] PADHI A K, NANJUNDASWAMY K S, GOODENOUGH G B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 1997, 144(4): 1188-1194.
[15] PROSINI P P, CAREWSKA M, SCACCIA S, WISNIEWSKI P, PASSERINI S, PASQUALI M. A new synthetic route for preparing LiFePO4 with enhanced electrochemical performance[J]. Journal of the Electrochemical Society, 2002, 149(7): A886-A890.
[16] CHANG Y C, SOHN H J. Electrochemical impedance analysis for lithium ion intercalation into graphitized carbons[J]. Journal of the Electrochemical Society, 2000, 147(1): 50-58.
基金项目:国家重点基础研究发展计划(973计划)资助项目(2007CB613607)
通讯作者:王志兴,教授,博士;电话/传真:0731-8836633;E-mail: wuling19840404@163.com


