文章编号:1004-0609(2008)02-0366-06
P204-P507在酸性硫酸盐溶液中对Nd3+和Sm3+的协同萃取
黄小卫1, 2, 3,李建宁2, 3,张永奇2, 3,龙志奇2, 3,王春梅2, 3,薛向欣1
(1. 东北大学 冶金资源与环境工程研究所,沈阳 110004;
2. 北京有色金属研究总院 稀土材料国家工程研究中心,北京 100088;
3. 有研稀土新材料股份有限公司,北京100088)
摘 要:
采用恒摩尔法、斜率法以及饱和容量法研究从硫酸介质中用P204(D2EHPA,用(HA)2表示)与P507(HEHEHP,用(HL)2表示)协同萃取Nd3+或Sm3+的性能与机理,并测定协萃反应的热力学平衡常数Ks.e,求得反应的焓变和熵变。结果表明,萃取反应呈现正协同效应,且协萃过程为放热反应,协萃配合物组成分别为Nd?(HA2)2?HL2和Sm?(HA2)2?HL2。因此,P204与P507混合使用可以改善硫酸介质下稀土的萃取分离效果。
关键词:
P204;P507;硫酸介质;协同萃取;Sm3+;Nd3+;
中图分类号:TF 845.6 文献标识码:A
Synergistic extraction of Nd3+ and Sm3+ from sulfuric acid medium with D2EHPA-HEHEHP in kerosine
HUANG Xiao-wei1, 2, 3, LI Jian-ning2, 3, ZHANG Yong-qi2, 3, LONG Zhi-qi2, 3,
WANG Chun-mei2, 3, XUE Xiang-xin1
(1. Institute of Metallurgical Resources and Environmental Engineering,
Northeastern University, Shenyang 110004, China;
2. National Engineering Research Center for Rare Earth Materials,
General Research Institute for Nonferrous Metals, Beijing 100088, China;
3. GRIREM Advanced Material Co., Ltd, Beijing 100088, China)
Abstract: The mechanism and characteristics about synergistic extraction of Nd3+ and Sm3+ by D2EHPA and HEHEHP from sulfuric acid medium were studied through the methods of slope, constant molecule and saturation extraction. The extraction equilibrium constants, enthalpy change and entropy change of the reaction were also calculated. The results show that the compositions of the extracted compound are identified as Nd?(HA2)2?HL2 and Sm? (HA2)2?HL2, the synergistic extractive effects are found, the extraction is an exothermic process. Consequently, the extraction and separation effect for rare earth can be improved with synergistic mixture of D2EHPA and HEHEHP.
Key words: D2EHPA; HEHEHP; sulfuric acid; synergistic extraction; Sm3+; Nd3+
协同萃取是有机溶剂萃取过程中很重要的现象,其应用已经涉及到很多领域,在稀土分离方面的研究也很多。李德谦等[1]研究了P507与HPMBP的苯溶液在不同酸介质中对La3+和Nd3+的协同萃 取,发现该混合萃取剂在硫酸、盐酸和硝酸介质中对La3+和Nd3+均有正协同效应。贾琼等[2]研究了HPMBP和中性有机磷(膦)类萃取剂Cyanex471X(TIBPS,B)在硝酸介质中对稀土元素La3+的萃取,并比较了HPMBP与Cyanex471X、Cyanex921、Cyanex923、Cyanex925、P507、P350及TBP的单独及混合体系萃取La3+的性能,发现所有混合体系对La3+均有正协同效应,其中HPMBP与Cyanex923、Cyanex921、Cyanex925组成的混合体系是萃取La3+的有效体系。
XIONG等[3]采用Cyanex272与P507在盐酸体系协同萃取重稀土元素,发现采用1.5 mol/L 50%Cyanex272+50%P507时,βHo/Dy与βEr/Ho比单独采用Cyanex272时小,而βYb/Tm和βLu/Yb比单独采用Cyanex272时大。
刘营等[4]研究了皂化Cyanex272和P507混合萃取剂对重稀土的萃取过程,发现与采用单一的P507相比,反萃酸度大大降低。
NAYAK等[5]采用P204和PC88A在盐酸介质中对钕和钷进行了协萃研究。
P204与P507是应用于稀土萃取分离方面两种常用的萃取剂。P204是一种中强酸性萃取剂,存在难以反萃、低酸下萃取容易乳化等不足,而P507是一种弱酸性萃取剂,相对P204则有容易反萃和酸性条件下萃取能力低等特点[6-8]。近年来,有研稀土新材料股份有限公司(稀土材料国家工程研究中心)利用上述这些特性,将P204与P507的协同萃取应用于稀土的萃取分离并取得了很好的效果[9-12]。虽然关于P204、P507与其他萃取剂对稀土的协同萃取机理研究报道较多[13-19],但是关于P204与P507二元体系在硫酸介质中对稀土的协同萃取机理的系统研究还未见报道。本文作者研究了P204与P507从硫酸介质中协同萃取Nd3+和Sm3+的机理,可为P204与P507混合萃取剂在工业上对稀土的萃取分离应用提供理论指导。
1 实验
所用仪器有:THZ-D台式恒温震荡器,PHS-3C精密pH计(上海雷磁仪器厂)。
所用P204和P507由洛阳市中达化工有限公司提供,经铜盐结晶纯化,纯度大于99%。煤油由北京北化永能化工有限公司提供,并经磺化处理。单一稀土溶液由纯度大于99.9%的单一稀土氧化物用稀硫酸溶解制得,并配成不同浓度和酸度。
将等体积的有机相和水相置于分液漏斗中,除温度实验外均在恒温操作箱(25±1)℃中,预热50 min,振荡30 min,静置,离心分相后,用EDTA络合滴定法测定水相中稀土浓度,有机相中稀土浓度用差减法求得;酸度用酸碱中和滴定法及pH计测定。
2 结果及讨论
取稀土浓度为0.1 mol/L的料液,用硫酸调节酸度,分别用纯P204、纯P507及P204-P507不同比例混合的有机相对稀土料液进行萃取平衡实验。
根据文献[20]中协萃系数的定义以及实验得出的各条件下的分配比计算出了由不同比例的P204与 P507组成的混合有机体系对Nd3+和Sm3+的协同萃取系数R,结果列于表1和表2。
表1 P507的比例对Sm3+协萃系数R的影响
Table 1 Synergistic enhancement coefficient (R) of Sm3+ in different pH values

表2 P507的比例对Nd3+协萃系数R的影响
Table 2 Synergistic enhancement coefficient (R) of Nd3+ in different pH values
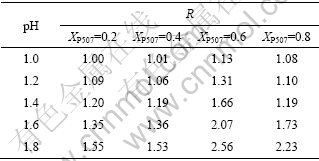
从表1和表2可以看出,不同比例的混合有机体系在硫酸介质中分别对Nd3+和Sm3+进行萃取时,协萃系数R均大于1,呈现不同程度的正协同效应,而且对Sm3+的正协同萃取效应比对Nd3+的明显。在同一比例混合有机体系下,协萃系数R随水相平衡酸度的增大而减小;在相同的水相平衡酸度下,当混合有机体系中P507为0.6时协萃系数R呈现最大值。
2.2.1 恒摩尔法
恒定有机相萃取剂P204和P507的总浓度为0.1 mol/L,分别固定Nd3+和Sm3+ 的浓度,改变平衡水相pH值以及混合有机相中P204与P507的比例,得到D12=DT-D1-D 2,其中DT表示混合体系的总分配比,D1和D2分别表示P204与P507单独萃取稀土的分配比。以lgD12对pH作图,其结果分别如图1和图2所示。
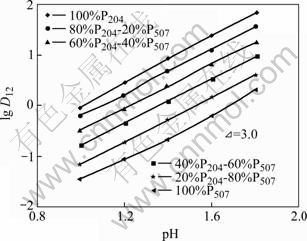
图1 平衡酸度对Sm3+分配比的影响
Fig.1 Acidity effect on distribution of Sm3+
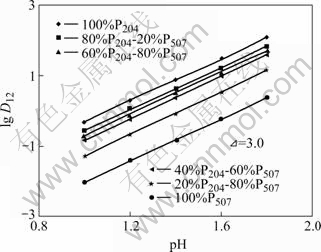
图2 平衡酸度对Nd3+分配比的影响
Fig.2 Acidity effect on distribution of Nd3+
从图1和图2可以看出,平衡水相的pH值越大,萃取Nd3+和Sm3+的分配比D值都逐渐增大。不管混合有机萃取体系中P507与P204的比例如何变化,萃取平衡曲线增加幅度基本一致,各条曲线基本平行,斜率都接近3。这说明在硫酸介质对Nd和Sm进行萃取时,纯P204、纯P507或两者的混合体系下,反应过程中都放出3个H+。
2.2.2 斜率法
用单浓度递变斜率法研究了P204-P507体系在硫酸介质中对Nd3+和Sm3+的协同萃取机理。假设P204和P507对Ln3+的协萃反应为:
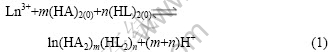
表观协萃平衡常数Ks.e为:

根据式(3),维持水相组成和有机相P507浓度不变,改变有机相P204的浓度,研究P204浓度变化对Nd3+和Sm3+协萃平衡的影响。结果如图3所示。从图3可以看出,lg D12-3pH与lg [P204]之间有良好的线性关系,所得直线斜率均接近2,即m=2。同理,维持水相组成和有机相P204浓度不变,改变有机相P507浓度,研究P507浓度变化对Nd3+和Sm3+协萃平衡的影响。结果示于图4。所得直线斜率均接近1,即n=1。根据电荷平衡原理以及图1、图2中直线的斜率,可得到m+n=3,由此推得Nd3+和Sm3+的协萃配合物组成分别为:Nd?(HA2)2?HL2和Sm? (HA2)2?HL2,协萃反应分别为:
![]()
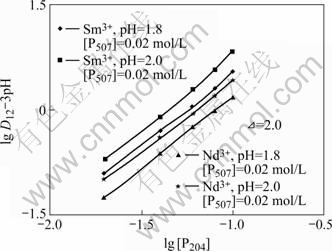
图3 lg D12-3pH与lg [P204]的关系
Fig.3 Relationship between lg D12-3pH and lg [P204]
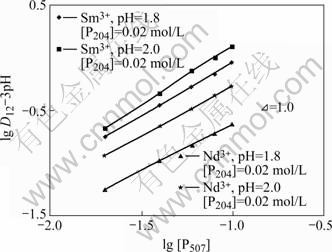
图4 lg D12-3pH与lg [P507]的关系
Fig.4 Relationship between lg D12-3pH and lg [P507]
2.2.3 饱和容量法
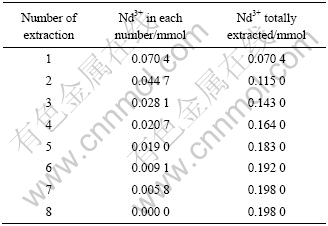
利用饱和容量法对P507与P204协同萃取Nd3+、Sm3+的机理进行验证,实验结果如表3和表4所列。经计算,Nd3+、P204和P507三者的摩尔比应为1?2?1;同样,Sm3+、P204和P507三者的摩尔比也应为1?2?1。结果与斜率法和恒摩尔法一致。
表3 P204与P507饱和萃取Sm3+的实验结果
Table 3 Results of saturated extraction of Sm3+ with P507(0.2 mmol) and P204(0.4 mmol)
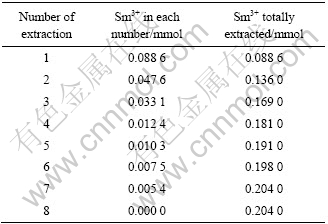
表4 P204与P507饱和萃取Nd3+的实验结果
Table 4 Results of saturated extraction of Nd3+ with P507(0.2 mmol) and P204(0.4 mmol)
在一定的水相和有机萃取剂浓度下,调节水相初始酸度,在25、30、35、40和45 ℃下进行萃取,求得不同温度下的协萃平衡常数以及lg Ks.e与温度的曲线(图5)。lg Ks.e的计算公式如下:
![]()
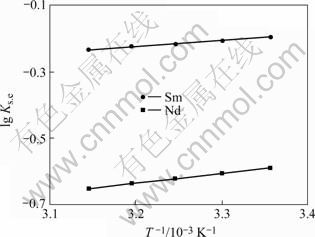
图5 协萃平衡常数与温度之间的关系
Fig.5 Relationships between equilibrium constant and temperature
表5所列分别为Nd3+和Sm3+离子协萃反应的焓变 (![]() )和熵变(
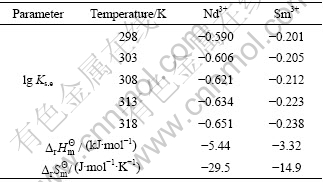
)和熵变(![]() )的计算结果。由表5可知,Nd3+和Sm3+离子反应的焓变和熵变均为负值,表明该协萃过程为放热反应,且协萃过程中Nd3+的焓变和熵变的绝对值大于Sm3+。
)的计算结果。由表5可知,Nd3+和Sm3+离子反应的焓变和熵变均为负值,表明该协萃过程为放热反应,且协萃过程中Nd3+的焓变和熵变的绝对值大于Sm3+。
表5 不同温度下P507和P204协萃Nd3+和Sm3+的热力学参数
Table 5 Thermodynamic parameters of Nd3+ and Sm3+ extraction with P507 and P204 at different temperatures
3 结论
1) P204-P507混合体系在硫酸介质中萃取Nd3+和Sm3+时具有正协同效应。萃取Sm3+ 的协同效应大于萃取Nd3+的协同效应,有利于Nd/Sm萃取分离,而且当混合萃取剂中P507的比例为60%时,正协同效应最大。
2) 确定了P204-P507协同萃取配合物组成比(摩尔比)Nd3+(Sm3+)?P204?P507为1?2?1,其萃合物的组成分别为Nd?(HA2)2?HL2和Sm?(HA2)2?HL2,其萃取机理可以表示为
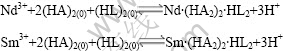
3) 得到了协萃反应的平衡常数Ks.e以及热力学常数![]() 和
和![]() ,表明协萃反应为放热过程。
,表明协萃反应为放热过程。
REFERENCES
[1] 李德谦, 牛 伟. HEHEHP与PMBP协同萃取稀土元素(Ⅲ)的机理研究[J]. 核化学与放射化学, 1990, 12(2): 92-98.
LI De-qian, NIU Wei. Investigation of synergistic extraction mechanism of rare earth elements(Ⅲ) with HEHEHP and PMBP[J]. Journal of Nuclear and Radiochemistry, 1990, 12(2): 92-98.
[2] 贾 琼, 李德谦, 牛春吉. 1-苯基-3-甲基-4-苯甲酰基-吡唑酮- 5与中性磷(膦)萃取剂协同萃取镧B[J]. 分析化学, 2004, 32(11): 1421-1425.
JIA Qiong, LI De-qian, NIU Chun-ji. Synergistic extraction of La B by mixtures (1-phenyl-3-methyl-4- benzoyl)-(pyrazolone-5) and neutral organphosphorus extractants[J]. Chinese Journal of Analytical Chemistry, 2004, 32(11): 1421-1425.
[3] XIONG Ying, WANG Xiang-lan, LI De-qian. Synergistic extraction and separation of heavy lanthanide by mixture of bis (2, 4, 4-trimethylpentyl) phosphinic acid and 2-ehtylhexyl phosphinic acid mono-2-ethylhexyl ester[J]. Separation Science and Technology, 2005, 40: 2325-2336.
[4] 刘 营, 邓佐国, 徐廷华. Cyanex272与HEHEHP混合体系萃取重稀土研究[J]. 稀有金属, 2000, 24(5): 394-397.
LIU Ying, DENG Zuo-guo, XU Ting-hua. Solvent extraction and separation of heavy rare earths with mixed Cyanex272 and HEHEHP[J]. Chinese Journal of Rare Metals, 2000, 24(5): 394-397.
[5] NAYAK D, LAHIRI S, DAS N R. Synergistic extraction of neodymium and carrier-free promethium by the mixture of HDEHP and PC88A[J]. Journal of Radioanalytical and Nuclear Chemistry, 1999, 210(2): 555-560.
[6] 徐光宪. 稀土(上)[M]. 北京: 冶金工业出版社, 1995: 503-540.
XU Guang-xian. Rare earth[M]. Beijing: Metallurgical Industry Press, 1995: 503-540.
[7] HUANG Xiao-wei, GU Bao-jiang, ZHANG Guo-cheng, CHEN Xiu-kun, ZHAO Chun-hui. Study on the process for removal of samarium from ndcl3 solution by solvent extraction with DEHPA[J]. Journal of Rare Earths, 1996, 15(2): 81-86.
[8] 黄小卫, 薛向欣, 李红卫, 张国成. 我国稀土湿法冶金发展状况及研究进展[J]. 中国稀土学报, 2006, 24(2): 129-133.
HUANG Xiao-wei, XUE Xiang-xin, LI Hong-wei, ZHANG Guo-cheng. Development status and research progress in rare earth hydrometallurgy in China[J]. Journal of the Chinese Rare Earth Society, 2006, 24(2): 129-133.
[9] 李建宁, 黄小卫, 朱兆武, 龙志奇, 彭新林, 崔大立. P204-P507-H2SO4体系萃取稀土元素的研究[J]. 中国稀土学报, 2007, 25(1): 55-58.
LI Jian-ning, HUANG Xiao-wei, ZHU Zhao-wu, LONG Zhi-qi, PENG Xin-lin, CUI Da-li. Extraction rare earth from D2EHPA-HEHEHP-H2SO4 system[J]. Journal of the Chinese Rare Earth Society, 2007, 25(1): 55-58.
[10] 黄小卫, 李建宁, 彭新林. 一种非皂化有机萃取剂萃取分离稀土元素的工艺[P]. CN1730680A, 2006-02-08.
HUANG Xiao-wei, LI Jian-ning, PENG Xin-lin. A method about extraction and separation of rare earth with unsaponificated extractant[P]. CN1730680A, 2006-02-08.
[11] 黄小卫, 李建宁, 彭新林. 一种非皂化磷类混合萃取剂萃取分离稀土元素的工艺[P]. CN1804063A, 2006-07-19.
HUANG Xiao-wei, LI Jian-ning, PENG Xin-lin. A method about extraction and separation of rare earth with unsaponificated mixed extractants[P]. CN1804063A, 2006-07-19.
[12] 黄小卫, 李红卫, 龙志奇. 一种非皂化有机相萃取稀土全分离工艺[P]. CN1824814A, 2006-08-30.
HUANG Xiao-wei, LI Hong-wei, LONG Zhi-qi. A method about extraction and separation of rare earth with unsaponificated extractant[P]. CN1824814A, 2006-08-30.
[13] TACHIMORI S, NAKAMURA H. Application of synergistic MEHPA-DEHPA mixed solvent to the extraction of lanthanides[J]. J Radioanal Nucl Chem, 1978, 44(1): 37-47.
[14] REDDY B V, REDDY L L, REDDY A S, REDY M L P, SUJATHA S, RAMAMOHANT R, DANODARAN A D. Synergistic solvent extraction of Eu and Tb with mixtures of various organophosphorus extractants[J]. J Radioanal Nucl Chem, 1994, 178(1): 109-120.
[15] KESHAVARZ A E, DARVISHI D, SADRNEZHAAD S K. Effect of TBP as a modifier for extraction of zinc and cadmium with a mixture of DEHPA and MEHPA[C]//Proceedings of the International Solvent Extraction Conference(Vol. 1). Cape Twon, South Africa, 2002: 1052-1057.
[16] BUCH A, STAMBOULI M, PAREAU D. Solvent extraction of nickel(Ⅱ) by mixture of 2-ethylhexanal oxime and bis(2- ethylhexyl) phosphoric acid[J]. Solvent Extr Ion Exch, 2002, 20(1): 49-66.
[17] 包福毅, 张文明. 石油亚砜和P507协同萃取稀土元素的研究[J]. 稀土, 1995, 16(2): 86-90.
BAO Fu-yi, ZHANG Wen-ming. Research on the synergistic extracting rare earth by the binary system of petroleum sulphoxide and P507[J]. Rare Earth, 1995, 16(2): 86-90.
[18] NAYAK D, LAHIRI S, DAS N R. Synergistic extraction of neodymium and carrier-free promethium by mixture of HDEHP and PC88A[J]. J Radioanal Nucl Chem, 1999, 240(2): 555-560.
[19] DHAMI P S, CHITNIS R R, GOPALAKRISHNAN V. Studies on the partitioning of actinides from high level waste using a mixture of HDEHP and CMPO as extractant[J]. Sci Tech, 2001, 36(2): 325-335.
[20] XU G X, WANG W Q, WU J. Extraction chemistry of the nuclear fuel(Ⅰ), Synergistic extraction with chelating and complexing extractants[J]. Atomic Energy Science and Technology, 1963, 7: 487-508.
基金项目:国家高技术研究发展计划资助项目(2006AA06Z123);国家自然基金资助项目(50674016)
收稿日期:2007-01-14;修订日期:2007-10-11
通讯作者:黄小卫,教授级高级工程师; 电话:010-82241180; E-mail: hxw0129@126.com


