文章编号:1004-0609(2010)08-1566-06
复合溶胶-凝胶法制备硅基含钛磷灰石蜂窝多孔涂层
吴振军1, 李文生1, 袁剑民2, 任艳群1, 陈宗璋1
(1. 湖南大学 化学化工学院,长沙 410082;2. 湖南大学 材料科学与工程学院,长沙 410082)
摘 要:
采用复合溶胶-凝胶法在单晶硅表面制备含钛磷灰石蜂窝状多孔涂层。采用热重-差热分析(TG-DSC)测定复合凝胶层转化为蜂窝多孔涂层的温度,采用SEM、ICP-AES、XRD与EDS等技术对涂层的微观形貌与成分进行分析;通过在pH值分别为7.0和7.4的模拟液(SBF)浸泡实验考察涂层的化学稳定性和对骨状磷灰石的诱导能力。结果表明:复合凝胶层转化为含钛磷灰石蜂窝多孔涂层的适宜温度为580~800 ℃,蜂窝状多孔涂层由含钛磷灰石构成,蜂窝孔径约为0.5~1.0 μm,涂层中贯穿有直径约100 nm、长数微米的氧化钛纳米线;蜂窝状多孔涂层在pH值为7.0的SBF中具有良好的化学稳定性,在pH值为7.4的SBF中能诱导骨状磷灰石的形核与生长,体现出优异的骨磷灰石诱导性能。
关键词:
中图分类号:TQ174.1 文献标志码:A
Fabrication of honeycomb-like titanium-containing apatite coating on silicon by hybrid sol-gel method
WU Zhen-jun1, LI Wen-sheng1, YUAN Jian-min2, REN Yan-qun1, CHEN Zong-zhang1
(1. College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China;
2. College of Materials Science and Engineering, Hunan University, Changsha 410082, China)
Abstract: The titanium-containing apatite coating (TCAC) with honeycomb-like structure was fabricated on single crystal silicon by a hybrid sol-gel approach including preparation of hybrid sol containing calcium, phosphorous and titanium, immersion and spin of specimens in hybrid sol, desiccation of hybrid sol, and calcination of hybrid gel. TG-DSC curve was used to determine appropriate temperature for the conversion of hybrid gel into coating. SEM, ICP-AES, XRD and EDS were employed to characterize the morphologies and compositions of specimens. The chemical stability and inductive ability for bone-like apatite of TCAC were tested by soaking specimens in simulated body fluid (SBF) with pH values of 7.0 and 7.4, respectively. The results show that hybrid gel forms honeycomb-like coating, which composes of titanium-containing apatite, at calcination temperatures ranging from 580 ℃ to 800 ℃, the aperture of coating is 0.5-1.0 μm and titania nanowires with approximately diameter of 100 nm and the micro-metric length are embedded in as-obtained honeycomb-like TCAC. TCAC possesses good chemical stability and bone-like apatite inductive ability based on nucleation and growth of apatite crystals in SBF with pH value of 7.0 and 7.4, respectively.
Key words: hybrid sol-gel; apatite; titania; honeycomb-like coating; single crystal silicon
单晶硅作为一种重要的半导体材料,已被广泛地应用于生物学和医学研究与应用领域中,目前,单晶硅在生物芯片、生物传感器和微电子机械系统等微型器件中都有重要的应用[1-4]。但晶体硅是一种生物惰性材料,被植入人体后,会导致凝血异常、炎症、组织病变等不良生理反应[5-6]。因此,对晶体硅进行表面生物学改性,提高晶体硅与细胞和组织的生物相容性,以期达到医学安全诊断和治疗的要求[7]。
目前,相关研究工作已开始关注硅的表面生物学改性,如采用离子注入方法在硅表层引入H等元素,使硅表面具备在生理环境中诱导沉积磷灰石生物涂层的能力[8]。另有报道分别采用化学沉积法[9-10]、溶胶-凝胶(sol-gel)法[11]和仿生沉积法[12]等在硅基表面直接制备磷灰石生物陶瓷涂层,但这些制备方法需采用后续高温处理提高磷灰石涂层的附着力,磷灰石的热膨胀系数在(13~18)×10-6 K-1,远高于晶体硅的热膨胀系数,这将导致磷灰石涂层内部及其与硅基界面出现残余应力,使涂层的力学稳定性下降。此外,前述方法制备的磷灰石涂层结构通常较为致密,植入体内后可能不利于细胞与新生组织的附着与生长。因此,引入热膨胀系介于磷灰石生物陶瓷涂层与硅基之间的组分,并在保持涂层具有优良生物相容性的前提下使其具有与基底相匹配的机械、力学性能以及适合细胞与组织附着和生长的表面结构,对扩大晶体硅在生物学和医学领域的应用具有重要的意义。
已有研究[13-21]表明,氧化铝、氧化锆以及氧化钛是提高生物陶瓷涂层力学性能的较理想的改性组分,特别是氧化钛还具有可靠的生物相容性。基于此,本研究通过制备同时含有钙、磷与钛的复合溶胶-凝胶(Hybrid sol-gel)体系,在单晶硅表面制备含钛的磷灰石蜂窝状多孔涂层,采用热重-差热技术(TG-DSC)确定含钛磷灰石涂层的形成温度,场发射扫描电镜(FE-SEM)观察涂层的形貌结构,电子能谱(EDS)和X射线衍射研究涂层的元素与晶体构成,在模拟体液(SBF)中浸泡样品以考察涂层的化学稳定性与诱导 能力。
1 实验
样品制备过程主要包括:
1) 硅片表面的清洗
先将厚度约为450 μm的单晶硅大圆片切割成 20 mm×10 mm的小片,分别用30%的盐酸和去离子水淋洗表面,再将硅片放在超声波清洗器中,用无水乙醇和丙酮分别清洗20 min后,最后用去离子水反复清洗表面,晾干备用。
2) 复合溶胶-凝胶的制备
精确称取1 g五氧化二磷(AR)和5.5 g四水硝酸钙(AR),在磁力搅拌下分别溶于30 mL无水乙醇(AR)中得到溶液A和溶液B,将溶液B在磁力搅拌下缓慢滴入溶液A中,陈化12 h,即得到含钙和磷的溶 胶(S1);
精确量取1.5 mL钛酸丁酯(AR),在磁力搅拌下溶入4.5 mL无水乙醇中,陈化12 h,即得到含钛的溶 胶(S2)。
将溶胶S1与溶胶S2在磁力搅拌下缓慢混合,陈化24 h得到含钛、钙与磷的复合溶胶(HS3)。
3) 硅基涂层的制备
先取一定量的复合溶胶HS3在80 ℃烘50 min,去除大部分溶剂形成复合凝胶(Hybrid gel,HG),采用综合热分析仪(STA449C,德国)对HG进行热重-差热(TG-DSC)分析,升温速率为10 ℃/min,测试在空气氛围中进行,确定HG转变为含钛磷灰石的适宜温度。
将准备的干净硅片放置在匀胶机吸盘中心,将复合溶胶HS3滴在硅基表面进行匀胶,形成硅基溶胶层,随后将硅基溶胶层在80 ℃保温50 min,得到硅基凝胶层,最后将硅基凝胶放入坩埚电阻炉(KSY-DT)中,在TG-DSC确定的含钛磷灰石烧成温度下处理1 h后随炉冷却,取出备测。
1.2 样品表征与体外性能评价
模拟体液(SBF)的配制见文献[22]。所配制的SBF成分相同,SBF的pH值采用缓冲液分别调节为7.0和7.4,SBF的pH值由精密数字pH计(PHS-3C,上海)测定。将制备好的硅基涂层在pH值不同的SBF中分别浸泡4 d和10 d。
采用等离子体原子发射光谱仪(ICP/AES,PS-6,美国;检出限10-3~10-4 ng/g级,精度约1%)测定浸泡4 d和10 d后pH为7.0的SBF中的钙、磷以及钛元素的含量。
采用SEM观察SBF浸泡前、后硅基涂层表面的微观形貌,采用面分析模式EDS测定涂层的元素构成,X射线衍射仪(XRD,Siemens D5000,CuKα1,35 kV,30 mA,λ=1.540 56 ?,德国)测定硅基涂层的物相。
2 结果与讨论
图1所示为复合凝胶的热重-差热分析(TG-DSC)结果。从图中1的TG曲线可知,在200 ℃左右,复合凝胶出现了明显的质量损失,质量减少量接近59%。在进行TG测试前,由于复合凝胶已在鼓风烘箱中于80 ℃干燥50 min,样品所含部分乙醇以及少量水等溶剂已基本挥发,因此,200 ℃附近的凝胶质量损失可能主要对应于凝胶中磷酸酯与钛酸丁酯燃烧和分解所放出的大量的CO2和H2O,以及硝酸钙分解释放出的氮氧化合物。因硝酸钙和钛酸丁酯分解温度较磷酸酯开始燃烧的温度低且需吸收大量的热,故在200 ℃附近的DSC曲线上首先出现一个向下的吸热峰,随着分解反应的完成,磷酸酯与钛酸丁酯分解产物开始燃烧并放出大量的热,从而在200~300 ℃出现一个明显的放热峰。从TG曲线上还可在500~600 ℃观察到一个较小的质量损失过程,这表明硝酸钙分解产物与磷酸酯燃烧产物之间发生反应生成磷灰石的同时,还有少量的副产物形成并挥发出来,通常生成磷灰石的反应为吸热过程,且副产物挥发也会带走一定的热量,因此在DSC曲线的相应位置出现一个较小的吸热峰。在温度高于600 ℃的区域,TG曲线上已没有明显的质量损失出现,但DSC曲线上有一个显著的吸热峰,这说明质量损失组分的燃烧、分解以及生成磷酸钙盐之外的副产物的反应已经完成,800 ℃附近的吸热峰对应于磷酸钙盐不同物相间的转变反应,如磷灰石的结晶及其转化为磷酸三钙等吸热化学变化过程。文献[11]中的研究表明:采用溶胶-凝胶体系制备磷灰石的焙烧温度高于800 ℃时,会有部分磷灰石热分解生成其它磷酸钙盐,其它磷酸钙盐在生理环境中的化学稳定性比磷灰石差,更重要的是会明显降低磷灰石涂层诱导骨组织附着与生长的性能。据此,采用复合溶胶-凝胶制备含钛磷灰石涂层的适宜烧结温度在580 ℃以上,但应低于800 ℃。为避免高温烧制过程晶体硅基与空气中的氧以及凝胶分解产物发生的反应所造成的对基材成分与性能的影响,本研究以600 ℃保温1 h作为含钛涂层的烧成工艺参数。
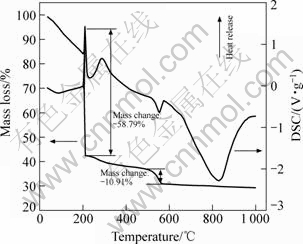
图1 复合凝胶的TG-DSC结果
Fig.1 TG-DSC results of as-prepared hybrid gel
图2所示为复合凝胶经600 ℃处理1 h及其在pH值为7.0的SBF中浸泡10 d和在pH值为7.4的SBF中浸泡4 d与10 d后的表面形貌。从图2可知,复合凝胶表面有直径为1~2 μm的开口与未开口的小孔,这应是复合溶胶中的乙醇和水等溶剂挥发所形成的(见图2(a));经600 ℃处理1 h后得到的涂层呈蜂窝状多孔结构(见图2(b)),孔径为0.5~1 μm,在大孔底部还可观察到10~50 nm的细孔。图2(c)所示为对应样品的放大照片,从该照片中还可观察到部分大孔边缘和内部出现了裂隙,并观察到了贯穿在蜂窝状多孔涂层中的直径约为10 nm的线状物(如图2c中箭头所示)。在通过干燥处理将复合溶胶转变为复合凝胶的过程中,主要为大部分乙醇溶剂的挥发,但在配制复合溶胶时,五氧化二磷与乙醇反应会生成一定量的水,加上硝酸钙水合物引入的水,在加热过程中会导致钛酸丁酯水解产生钛酸。相关研究[23-24]表明,当加热到一定温度时,钛酸会逐渐失水首先生成薄膜状的非定形氧化钛,进一步升高温度,薄膜状氧化钛则卷曲形成氧化钛纳米管或氧化钛纳米线,且在碱性环境中更适于氧化钛一维纳米结构的形成。本研究所制备的复合凝胶在焙烧过程中,硝酸钙热分解得到的氧化钙及其进一步与磷酸根反应生成的磷灰石都是碱性物质,因此图2(c)中贯穿于涂层中的纳米线状物可能为氧化钛一维纳米材料。
在pH值为7.0的SBF中浸泡10 d后,所形成的蜂窝状多孔涂层的结构未发生明显变化(见图2(d)),绝大部分孔洞孔径为500 nm左右,且仍可观察到直径10 nm左右的线状物,其形态也没有明显变化。这表明所制备的蜂窝状多孔涂层在pH值较低仿生溶液中具有较好的化学稳定性。在pH值为7.4的SBF中分别浸泡4 d与10 d后,涂层的表面形貌如图2(e)和(f)所示。从图2(e)和(f)可知,在浸泡4 d的样品表面形成了100 nm以下的粒状突出物,这与文献[25]报道的SBF中钙、磷元素富集形核的形态相近;浸泡10 d后在样品表面形成了大量由30~50 nm的针状晶体构成的新沉积层,这种结构也与骨状磷灰石结构相似[26-28],表明所制备的蜂窝状多孔涂层在SBF中能有效地诱导磷灰石类似物的形核与生长。
图3所示为所制备的硅基蜂窝状多孔涂层在pH值不同的SBF中浸泡10 d前后的EDS谱。从图3可知,除硅之外,EDS还检测出涂层含有钙、磷以及钛元素,其中钙和磷的摩尔比约为1.66?1,接近原料以及化学整比羟基磷灰石中的钙与磷的摩尔比(为1.67?1),钙和钛的摩尔比约为5.5,也与原料的配比相近。这表明在600 ℃下,复合凝胶中的钙、磷和钛均不会出现明显的热挥发损失。与浸泡前相比,在pH为7.0的SBF中浸泡10 d后(见图3(b)),样品的EDS谱差异不明显,且相应的ICP-AES测试也表明SBF中的钙、磷、钛浓度没有可检出的变化,这进一步证实所制备的硅基蜂窝状涂层在较低pH值的SBF中具有较优的化学稳定性。图3(c)所示为样品在pH为7.4的SBF中浸泡10 d后的EDS谱,可观察到明显增强的钙、磷元素峰,基底硅的峰则有所减弱,表明有含钙和磷的新物质在硅基蜂窝状涂层表面形成,这与SEM的结果一致(见图2(f))。
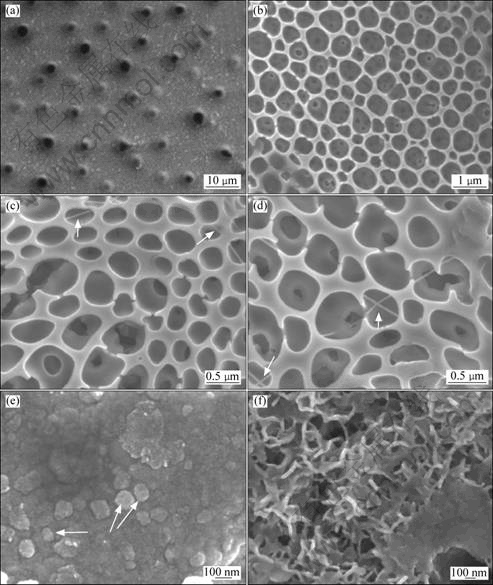
图2 不同条件下制备样品的形貌
Fig.2 Morphologies of specimens subjected to different processes: (a) Hybrid gel; (b) Coating formed by calcination at 600 ℃ for 1 h; (c) Magnified photo of (b); (d) Soaking in SBF with pH of 7.0 for 10 d; (e) Soaking in SBF with pH of 7.4 for 4 d; (f) Soaking in SBF with pH of 7.4 for 10 d
图4所示为硅基蜂窝状多孔涂层在pH值为7.0与7.4的SBF中浸泡前、后的XRD谱。从图4中可以看出,各条谱线在2θ为31~35?、62?均有明显的磷灰石所特有的衍射峰出现,2θ=70?则对应单晶硅的衍射峰,这表明在600 ℃热处理后,晶体硅表面的复合凝胶层已转化为磷灰石涂层。与基底硅的衍射峰相比,磷灰石在2θ为31~33?附近的三强衍射峰相对较弱且未清晰分离,这可归因于蜂窝多孔涂层的结晶性相对较低、构成涂层的晶体具有纳米尺寸造成的衍射峰宽化。而衍射谱中未出现氧化钛的衍射峰,这可能是因为氧化钛晶体在复合涂层中的含量较低且相对分散所致。在pH值为7.0的SBF中浸泡10 d后,与浸泡前对比发现谱没有明显变化,而在pH值为7.4的SBF中分别浸泡4 d和10 d后,磷灰石的特征衍射峰明显增强,不仅在2θ为20~25?附近出现了宽化的新的衍射峰,在2θ为31~35?的磷灰石三强特征衍射峰也分离得更加清晰,涂层衍射峰强度的改变证实所制备的硅基蜂窝状多孔含钛磷灰石涂层在SBF中具有优异的诱导骨状磷灰石形核与生长的性能。
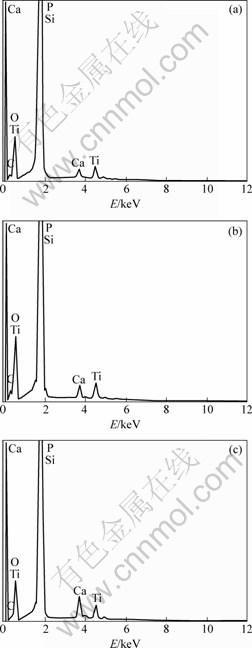
图3 经600 ℃烧制1 h 获得的涂层(a)及其在pH值分别为7.0(b)和7.4(c)的SBF中浸泡10 d后的EDS谱
Fig.3 EDS spectra of coating after calcination at 600 ℃ for 1 h (a) and then soaking in SBF with pH of 7.0 (b) and 7.4 (c) for 10 d
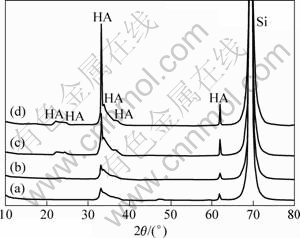
图4 在600 ℃烧制1 h所制备的硅基涂层及其在pH值为7.0的SBF中浸泡4 d和在pH值为7.4的SBF中浸泡4 d与10 d后的XRD谱
Fig.4 XRD patterns of coating after calcination at 600 ℃ for 1 h (a) and then soaking in SBF with pH of 7.0 for 4 d (b) and pH of 7.4 for 4 d (c) and 10 d (d)
3 结论
1) 采用涂覆和焙烧含钙、磷与钛的复合溶胶-凝胶体系在单晶硅基表面制备含钛的磷灰石复合涂层。
2) 复合溶胶在溶剂挥发和酯水解共同作用下,使所制备的硅基含钛磷灰石复合涂层形成蜂窝状多孔结构,蜂窝结构的孔径为0.5~2 μm;其中,钛酸丁酯的水解以及碱性焙烧环境可能是导致在蜂窝涂层形成直径约10 nm、长度为微米级的纳米线状物,其成分应为氧化钛。
3) 硅基含钛磷灰石复合涂层在pH值为7.0的SBF中出现溶解,多孔磷灰石与氧化钛纳米线状结构在中性SBF中具有良好的化学稳定性;在pH值为7.4的SBF中浸泡4 d后,其表面能诱导纳米磷灰石形核,浸泡10 d后,能诱导沉积形成骨状纳米磷灰石晶体。这表明硅基含钛蜂窝状多孔磷灰石涂层具有优异的骨磷灰石诱导性能。
REFERENCES
[1] DIAO J, REN D, JAMES R, LEE K. A surface modification strategy on silicon nitride for developing biosensors[J]. Anal Biochem, 2005, 343(2): 322-328.
[2] BESSUEILLE F, DUGAS V, VIKULOV V, CLOAREC J, SOUTEYRAND E, MARTIN J. Assessment of porous silicon substrate for well-characterised sensitive DNA chip implement[J]. Biosensors and Bioelectronics, 2005, 21(6): 908-916.
[3] ARCHER M, CHRISTOPHERSEN M, FAUCHET P M. Electrical porous silicon chemical sensor for detection of organic solvents[J]. Sensors and Actuators B, 2005, 106(1): 347-357.
[4] ZAURIA S, MARTELET C, JAFFREZIC R, MGAIETH R, MAAREF H, LAMARTINE R. Porous silicon a transducer material for a high-sensitive biochemical sensor: Effect of a porosity, pores morphologies and a large surface area on a sensitivity[J]. Thin Solid Films, 2001, 383(1/2): 325-327.
[5] BHARAT B, DHARMA R T, MATTHEW T K, STEPHEN C L. Morphology and adhesion of biomolecules on silicon based surfaces[J]. Acta Biomater, 2005, 1(3): 327-341.
[6] SUET P L, WILLIAMS K A, CANHAM L T, NICOLAS H V. Evaluation of mammalian cell adhesion on surface-modified porous silicon[J]. Biomaterials, 2006, 27(6): 4538-4546.
[7] DING Yan-jun, LIU Jia, WANG Hua, SHEN Guo-li, YU Ru-qin. A piezoelectric immunosensor for the detection of α-fetoprotein using an interface of gold/hydroxyapatite hybrid nanomaterial[J]. Biomaterials, 2007, 28(12): 2147-2154.
[8] LIU X Y, FU R K, POON R W, CHEN P, CHU P K, DING C X. Biomimetic growth of apatite on hydrogen-implanted silicon[J]. Biomaterials, 2004, 25(25): 5575-5581.
[9] CHEN S, ZHU Z, ZHU J, ZHANG J, SHI Y, YU K, WANG W, WANG X, FENG X, LUO L, SHAO L. Hydroxyapatite coating on porous silicon substrate obtained by precipitation process[J]. App Surf Sci, 2004, 230(1/4): 418-424.
[10] CHUNG R, HSIEH M, PANDA R, CHIN T. Hydroxyapatite layers deposited from aqueous solutions on hydrophilic silicon substrate[J]. Surf Coat Technol, 2003, 165(2): 194-200.
[11] HWANG K, LIM Y. Chemical and structural changes of hydroxyapatite films by using a sol-gel method[J]. Surf Coat Technol, 1999, 115(2): 172-175.
[12] HATA K, OZAWA N, KOKUBO T. Mechanism of apatite formation on single crystal silicon in an aqueous solution at room temperature[J]. J Ceram Soc Jpn, 2002, 110(11): 990-994.
[13] SHI D L, JIANG G W. Synthesis of hydroxyapatite films on porous Al2O3 substrate for hard tissue prosthetics[J]. Mater Sci Eng C, 1998, 6(3): 175-182.
[14] 吴振军, 杨 妍, 陈宗璋, 李素芳. 硅基HA/Al2O3复合生物涂层的制备与表征[J]. 功能材料, 2008, 39(8): 1355-1358.
WU Zhen-jun, YANG Yan, CHEN Zong-zhang, LI Su-fang. Fabrication and characterization of HA/Al2O3 composite biocoating on silicon[J]. Chin J Funct Mater, 2008, 39(8): 1355-1358.
[15] 王周成, 倪永金, 黄金聪. 电泳沉积和反应结合制备羟基磷灰石/氧化铝复合涂层[J]. 硅酸盐学报, 2008,36(6): 71-76.
WANG Zhou-cheng, NI Yong-jin, HUANG Jin-cong. Fabrication of hydroxyapatite/aluminum oxide composite coatings by the combination of electrophoretic deposition and reaction bonding process[J]. J the Chin Ceram Soc, 2008,36(6): 71-76.
[16] 宁成云, 王迎军, 叶建东, 赵娜如, 陈晓峰, 吴 刚. 氧化锆增韧HA/ZrO2功能梯度涂层的TEM分析[J]. 功能材料, 2006, 37(3): 69-71.
NING Cheng-yun, WANG Ying-jun, YE Jian-dong, ZHAO Na-ru, CHEN Xiao-feng, WU Gang. TEM study on HA/ZrO2 functional gradient coatings reinforenced by ZrO2[J]. Chin J Funct Mater, 2006, 37(3): 69-71.
[17] XIAO X F, LIU R F, ZHENG Y Z. Characterization of hydroxyapatite/titania composite coatings codeposited by a hydrothermal–electrochemical method on titanium[J]. Surf Coat Technol, 2006, 200(14/15): 4406-4413.
[18] YAMASHITA K, NAGAI M, UMEGAKI T. Fabrication of green films of single- and multi-component ceramic composites by electrophoretic deposition technique[J]. J Mater Sci, 1997, 32(12): 6661-6664.
[19] ISHIZAWA H, OGINO M. Characterization of thin hydroxyapatite layers formed on anodic titanium oxide films containing Ca and P by hydrothermal treatment[J]. J Biomed Mater Res, 1995, 29(6): 1071-1079.
[20] KUMAR R, WANG M. Functionally graded bioactive coatings of hydroxyapatite/titanium oxide composite system[J]. Mater Lett, 2002, 55(3): 133-137.
[21] 付 涛, 徐可为, 憨 勇. 等离子喷涂-水热处理制备二氧化钛-羟基磷灰石复合涂层[J]. 硅酸盐学报, 2003,31(1): 95-98.
FU Tao, XU Ke-wei, HAN Yong. Titania-hydroxyapatite composite coating prepared by plasma spray-hydrothermal treatment[J]. J the Chin Ceram Soc, 2003,31(1): 95-98.
[22] 吴振军, 何莉萍, 陈宗璋. 含钙阳极氧化铝的制备及其体外性能[J]. 中国有色金属学报, 2005, 15(10): 1572-1576.
WU Zhen-jun, HE Li-ping, CHEN Zong-zhang. Fabrication and in vitro performance of anodic alumina containing calcium[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(10): 1572-1576.
[23] KASUGA T, HIRAMATSU M, HOSON A, SEKONO T, NIIHARA K. Formation of titanium oxide nanotube[J]. Langmuir 1998, 14(12): 3160-3163.
[24] 孔祥荣, 彭 鹏, 孙桂香, 郑文君. 二氧化钛纳米管的研究进展[J]. 化学通报, 2007, 70(1): 10-15.
KONG Xiang-rong, PENG Peng, SUN Gui-xiang, ZHENG Wen-jun. Research advances in TiO2 nanotubes[J]. Chemistry, 2007, 70(1): 10-15.
[25] TSURU K, KUBO M, HAYKAMA S, OHTSUKI C, OSAKA A. Kinetics of apatite deposition of silica gel dependent on the inorganic ion composition of simulated body fluids[J]. J Ceram Soc Jpn, 2001, 109(5): 412-418.
[26] LENKA J, FRANK A M, ALES H, JAKUB S, PETER G. Biomimetic apatite formation on chemically treated titanium[J]. Biomaterials, 2004, 25(3): 1187-1194.
[27] UCHIDA M, KIM H M, KOKUBO T, TANAKA K, NAKAMURA T. Structural dependence of apatite formation on zirconia gels in a simulated body fluid[J]. J Ceram Soc Jpn, 2002, 110(8): 710-715.
[28] SHIRTLIFF V J, HENCH L L. Bioactive materials for tissue engineering, regeneration and repair[J]. J Mater Sci, 2003, 38(3): 4697-4707.
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(50702020)
收稿日期:2009-09-27;修订日期:2010-04-20
通信作者:吴振军,副教授,博士;电话:0731-88821017;E-mail:wooawt@yahoo.com.cn


