
Fabrication of transparent YAG ceramics bytraditional solid-state-reaction method
LI Chang-qing(李长青), ZUO Hong-bo(左洪波), ZHANG Ming-fu(张明福),
HAN Jie-cai(韩杰才), MENG Song-he(孟松鹤)
Center for Composite Materials, Harbin Institute of Technology, Harbin 150001, China
Received 3 March 2006; accepted 30 November 2006
Abstract:
Transparent polycrystalline YAG ceramics were fabricated by solid-state reaction method using commercial ultrafine yttria and α-Al2O3 powders. The starting materials were milled and calcined at 1 400 ℃, and sintered into transparent YAG ceramics at 1 750 ℃ in the vacuum for 4 h. Neither the starting materials as-milled or those calcined into YAG phase at 1 500 ℃ can be sintered into transparent ceramics. Wide grain boundaries emerge in the YAG ceramics sintered at 1 850 ℃ for 4 h, at the edge of which YAG phases decompose into perovskite YAlO3(YAP) and α-Al2O3.
Key words:
YAG; α-alumina; yttria; perovskite; sintering;
1 Introduction
Yttrium aluminum garnet (Y3Al5O12, YAG) belongs to the space group Ia3d(Oh10) and its unit cell consists of eight formula units with 160 atoms. YAG is a candidate for applications of high-temperature structural materials and refractory coatings for electronic devices due to its low creep rate, high thermal stability and good chemical resistance, and it is widely used in lasers as a host for rare-earth-based phosphors[1-2]. Researchers have attempted to synthesize transparent YAG ceramics by several methods, but it is expensive and difficult to produce large size YAG single crystals. DE WITH and VON DIJK[3] prepared transparent YAG samples via vacuum sintering at 1 850 ℃ for 4 h using YAG powders synthesized by a sulfate co-pyrolysis method. LI et al[4] produced YAG powders via co-precipitation method. IKESUE and FURUSATO[5] fabricated polycrystalline transparent YAG ceramics by solid-state reaction method using high-purity α-Al2O3 and Y2O3 powders synthesized by the pyrolysis of Y2(OH)Cl5?nH2O and Al(OH)3. WEN et al[6], WANG et al[7-8] fabricated transparent YAG ceramics by solid-state reaction method using yttria produced by a wet chemical method and commercial α-Al2O3.
Most transparent YAG ceramics are fabricated by calcining starting materials into pure YAG phase and then sintering into YAG ceramics. In the present work, YAG ceramics were synthesized by solid-state reaction method using commercial ultra fine yttria and α-Al2O3 powders as starting materials. Microstructure and optical properties of as-prepared YAG ceramics were also studied.
2 Experimental
The starting materials were commercially available aggregated α-alumina and yttria (4 N grade, Shanghai Reagent Co., China). The starting powders were milled in analytical ethanol added with tetraethyl silicate (0.5%, mass faction, as for starting powders) for 12 h. The milled slurry was dried, and then calcined at different temperatures in air for 5 h. The calcined powder was analyzed by using Rigaku D/Max RB XRD diffractometer with Cu Kα(XRD) and JEOL JEM- 1200EX transmission electron microscope(TEM). The calcined powder was pressed into pellets and sintered at 1 750 ℃ for 4 h under vacuum of 7×10-3 Pa. Sintered samples were polished on both sides and used for measuring optical transmittance with 7U-1901 UV-VIS spectrophotometer over wavelength of 190-900 nm and Perkin Elmer Spectrum One FT-IR spectrometer over wavelength of 1.33-10 mm. The microstructure and compositions of sintered samples were observed by HITACHI series scanning electronic microscope(SEM) and analyzed by XRF-1800 sequential X-ray fluorescence spectrometer(XRF), respectively.
Table 1 shows the different specifications of specimens in the present work. Sample B was not calcined before the mixed powder was pressed into pellet and sample D was vacuum-sintered at 1 850 ℃ much higher than other three samples.
Table 1 Specification of samples in present work

3 Results and discussion
Fig.1 shows the X-ray diffraction patterns of the mixed powders calcined at different temperatures. The milled XRD profile shows no reaction occurred between Y2O3 and α-Al2O3 phases. There are YAG, YAlO3(YAP), Y4Al2O9(YAM), Y2O3, and α-Al2O3 phases in the mixed powder calcined at 1 300 ℃ and 1 400 ℃. Another two X-ray diffraction patterns indicate that powder calcined at 1 450 ℃ is composed of most YAG and some YAM phases, while the powder calcined at 1 500 ℃ is almost YAG phase within the detection limits of XRD. For no reaction occurs in as-milled mixture, yttria peaks are much stronger than those of α-Al2O3 and the mixture remains in crystalline structure. The stability of the crystal structure of the materials depends on their inherent nature. When α-Al2O3 and yttria mix together, yttria acts as soft materials while α-Al2O3 acts as hard materials, so yttria is not easy to grind. Yttria peaks are relatively weaker than those of α-alumina[9], and both of them do not transform into amorphous states.
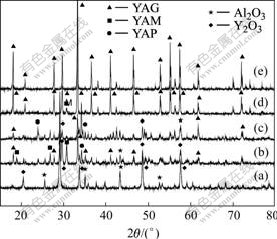
Fig.1 X-ray diffraction patterns of mixed powders calcined at different temperatures: (a) As-milled; (b) 1 300 ℃; (c) 1 400 ℃; (d) 1 450 ℃; (e) 1 500 ℃
Fig.2 shows TEM micrographs of α-Al2O3 and yttria powders as starting materials. The powders are aggregated, and their primary particles are in sub- micrometers.
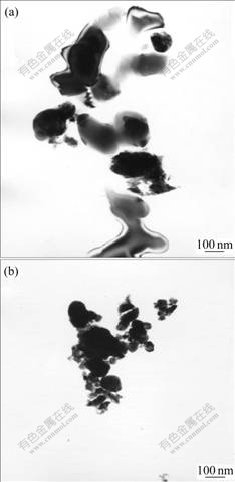
Fig.2 TEM micrographs of starting materials: (a) Yttria; (b) α- Al2O3
Fig.3 shows TEM micrographs of two specifications of mixed powders. Fig.3(a) is TEM micrograph of as-milled powder and Fig.3(b) is milled powder calcined at 1 400 ℃. Both of them belong to sub-micrometer particles. The as-milled powder is so active that it sticks to mortar handle easily during the course of grinding, as reported by ROOSEN and HAUSNER[10]. And the activity is not distinct for the powders calcined at 1 500 ℃ or milled with low ball to powder ratio, which maybe indicates high sinterability and defective energy due to high energy ball milling.
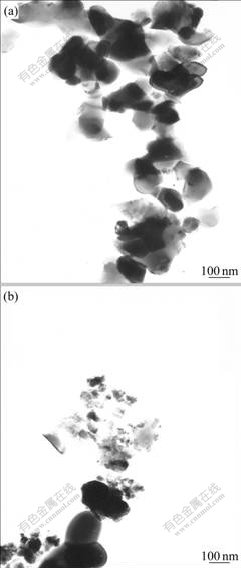
Fig.3 TEM micrographs of powder sample: (a) As-milled powder; (b) Milled powder calcined at 1 400 ℃
Fig. 4 shows the appearances samples of A, B, C, D sintered in the vacuum described in Table 1. Sample A is examined to have much different color from samples B and C, and more transparent than D judged by visual detection. Sample B is dark and sample C is white. Sample D is translucent and more fragile to crack than sample A during polishing. The sintering shrinkage rates of samples A, B, C, D are 17.2%, 16.3%, 8.4%, 13.2%, respectively.

Fig.4 Appearance of samples sintered in vacuum (specification described in Table 1)
Fig.5 shows the on-line transmittance spectra for polished sample A (0.80 mm in thickness). Optical transmittance is one of the major parameters for evaluating the optical properties of transparent ceramics. Optical transmittance of sample A is about 40% in the visible region, up to above 77% ranging from 1.33 mm to 4.8 mm, even above 80% between 4.8 mm and 6 mm that is near the maximum theoretical transmittance. The fact that the data of optical transmittance of YAG ceramics in visible region are lower than those of IKESUE[5] and WEN[6] may be due to not long enough vacuum sintering time.
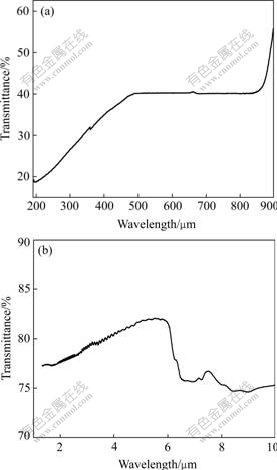
Fig.5 Optical transmittance of YAG ceramics (sample A)
Fig.6 shows the relationship of relative density and temperature for samples sintered from 1 500 ℃ to 1 750℃ in vacuum using milled powder calcined at 1 400℃. The shape of curve is similar to that RAHAMAN[11] described, and the maximum sintering shrinkage rate takes place between 1 500 ℃ and 1 600℃. The high activity of milled powder calcined at 1 400 ℃ is further confirmed and relative density of sample sintered at 1 750 ℃ could reach 99.95%. Below 1 500 ℃, sintering shrinkage rate is low, which may be due to: 1) not enough activity under low sintering temperature; 2) relieved transient stress by creep processes because of different densities of different regions of the compact [11].
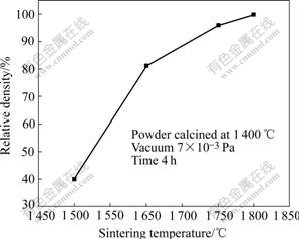
Fig.6 Relationship between relative density and sintering temperature of samples
Table 2 lists the compositions of different samples. Theoretical value of YAG suggests that no Y2O3, α-Al2O3 or SiO2(from TEOS) is volatile during high temperature sintering. The composition of powder calcined at 1 400 ℃ and sample A are valued by XRF that has a high sensitive degree. There are some content deviations between theoretical and analyzed data in sample A and powder calcined at 1 400 ℃. Maybe there are two reasons: some impurities come from process of experiment and starting materials; content in ceramics may be volatile a little during sintering, especially during high temperature vacuum sintering. Since Y2O3, α-Al2O3 contents in YAG do not deviate from stoichiometric compound by over 2%, as seen in Table 2, and KUKLJA [12] confirmed that excess Y2O3 up to 2% and surplus α-Al2O3 only up to 0.5% could exist in YAG, Si could take place of Al ion in Al-O tetrahedron in YAG; and antisite disorder is the main mechanism in YAG and prefers over the Frenkel and Schottky-like disorder. Extra Y ions can be accommodated in YAG lattice and more easily replace Al ions in octahedral position. During high temperature sintering, for different melting points of Y2O3 and α-Al2O3, volatile amount of these two contents will be different, and it is difficult to control precise stoichiometric contents of Y2O3, α-Al2O3 in YAG ceramics.
Table 2 Composition of different samples (mass fraction, %)
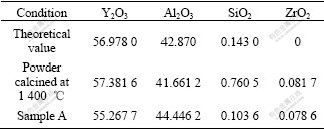
Fig.7 shows surface and fracture surface morphologies of sample A by SEM. Fig.7(a) shows the surface morphology after chemical etching and Fig.7(b) shows the fracture surface of sample A. The sample consists of uniform grains with about 10 μm in dimension, and some little holes can be revealed in the grain.
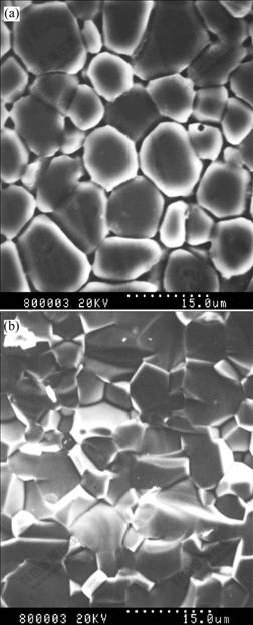
Fig.7 SEM photographs of sample A: (a) Surface after chemical etching; (b) Fracture surface
Fig.8 indicates the microstructures of samples B, C after vacuum sintering evaluated by SEM. SEM image of sample B shows that some position is melted locally for there is some impurity, as can be seen in Fig.8(a). Perhaps impurities in the samples could not escape from ceramic pellet inside completely under vacuum sintering, some impurities are volatile and others are carbonated into dark particles during high temperature vacuum sintering. SEM image of sample C, as can be seen in Fig.8(b), shows a white ceramic pellet different from sample A. Grain size of sample C is about 1-3 μm. Most spherical particles adjoin together through neck formation among the adjacent particles, and holes are left inside. Sinterability of sample C is so low that it could not be sintered into high density ceramics.

Fig.8 Surfaces of samples observed by SEM: (a) Sample B; (b) Sample C
Fig.9 shows the SEM morphology of sample D sintered at 1 850 ℃ in vacuum. Grain size of sample D is much larger than that of sample A, even larger than 100 mm. The grain boundary is much wider than that of sample A, and the dimension of grain boundary of sample D as seen in Fig.9(a) is wider than 100 mm. Chemical composition of grain boundary is valued by EDX. Fig.9(b) shows that yttrium element is deficient and aluminum is rich compared with that of the grain composition in Table 2. Grain boundary is maybe composed by eutectic type of structure such as perovskite structure YAP and α-Al2O3 phases. The similar situation was confirmed by COCKAYNE and LENT[13], WANG[14-15] and PRADHAN et al[16]. The energy of YAG grain boundary is much higher than that of crystal grain, so YAG phase on the grain boundary would decompose into YAP and α-Al2O3, as expressed by the following reaction[13]:
Y3Al5O12→3YAlO3+Al2O3 (1)

Fig.9 SEM morphology(a) and EDX spectra(b) of grain boundary of sample D
The transparency of high temperature sintered ceramics for sample D is lower than that of sample A distinctly judged by visual detection, maybe because the refractive index of α-Al2O3 (ne=1.760, no=1.768) and YAP (1.96) on grain boundary are different from those of YAG (no=1.815) in grain. There are some second phase particles in crystal grain, which act as scattering centers and make transmittance decreased.
4 Conclusions
1) Transparent YAG ceramics could be synthesized by using commercial α-Al2O3 and Y2O3 powders calcined at 1 400 ℃ and sintered at 1 750 ℃ in vacuum.
2) YAG powder calcined at 1 500 ℃ could not be sintered into transparent ceramics because the activity of calcined YAG powder is not high enough.
3) At higher sintering temperature as 1 850 ℃, grain size of YAG ceramics enlarges unusually and grain boundary grows much wide. At the edge of the grain boundaries, YAP and α-Al2O3 phases maybe decompose by YAG grain, which results in the decrease of the transmittance of oversintered YAG ceramics.
References
[1] SHOJI I, KURIMURA S, SATO Y, TAIRA T, IKESUE A, YOSHIDA K. Optical properties and laser characteristics of highly Nd3+-doped Y3Al5O12 ceramics [J]. Appl Phys Lett, 2000, 77(7): 939-941.
[2] PARK C H, PARK S J, YU B Y, BAE H S, KIM C H, PYUN C H. VUV excitation of Y3Al5O12: Tb phosphor prepared by a sol-gel process [J]. J Mater Sci Lett, 2000, 19(4): 335-338.
[3] DE WITH G, VAN DIJK H J A. Translucent Y3Al5O12 ceramics [J]. Material Research Bulletin, 1984, 19: 1669-1674.
[4] LI J G, IKEGAMI V, LEE J H. Low-temperature fabrication of transparent yttrium aluminum garnet (YAG) ceramics without additives [J]. J Am Ceram Soc, 2000, 83(4): 961-963.
[5] IKESUE A, FURUSATO I. Fabrication of polycrystalline, transparent YAG ceramics by a solid-state reaction method [J]. J Am Ceram Soc, 1995, 78(1): 225-228.
[6] WEN Lei, SUN Xu-dong, XIU Zhi-meng, CHEN Shao-wei, TSAI C T. Synthesis of nanocrystalline yttria powder and fabrication of transparent YAG ceramics [J]. Journal of the European Ceramic Society, 2004, 24(9): 2681-2688.
[7] WANG Jie-qiang, YUE Yun-long, TAO Wen-hong, YU Qing-hua, TAO Zhen-dong, SUN Xu-dong. Preparation of transparent yttrium aluminum garnet ceramics by relatively low temperature solid state reaction [J]. Trans Nonferrous Met Soc China, 2003, 13(5): 1096-1101.
[8] WANG Jie-qiang, TAO Zhen-dong, ZHENG Shao-hua, HAO Zhe, XUN Xu-dong. Influence of fabrication conditions on transparency of YAG polycrystalline by solid state reaction method [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(2): 432-436. (in Chinese)
[9] ZHANG Qi-wu, SAITO F. Mechanochemical solid reaction of yttria with alumina leading to the synthesis of yttrium aluminum garnet [J]. Powder Technology, 2003, 129(1/3): 86-91.
[10] ROOSEN A, HAUSNER H. Techniques for agglomeration control during wet-chemical powder synthesis [J]. Advanced Ceramic Materials, 1988, 3(2): 131-137.
[11] RAHAMAN M N, DE JONGHE L C, CHU M Y. Effect of green density on densification and creep during sintering [J]. J Am Ceram Soc, 1991, 74(3): 514-519.
[12] KUKLJA M M. Defects in yttrium aluminium perovskite and garnet crystals: Atomistic study [J]. J Phys Matter, 2000, 12(13): 2953-2967.
[13] COCKYNE B, LENT B. A Complexity in the solidification behaviour of molten Y3Al5O12 [J]. Journal of Crystal Growth, 1979, 46(3): 371-379.
[14] WANG S, YAMAMOTO F, AKATSU T, TANABE Y, YASUDA E. Metastable precipitation of YAlO3 in isothermally solidified YAG/Al2O3-rich spinel composites [J]. Journal of Materials Science, 1998, 33(21): 5157-5162.
[15] LIN I C, NAVROTSKY A, RICHARD WEBER J K, NORDINE P C. Thermodynamics of glass formation and metastable solidification of molten Y3Al5O12 [J]. Joural of Non-Crystalline Solids, 1999, 243(2/3): 273-276.
[16] PRADHAN A K, ZHANG KAI, LOUTS G B. Synthesis of neodymium-doped yttrium garnet(YAG) nanocrystalline powders leading to transparent ceramics [J]. Material Research Bulletin, 2004, 39(9): 1291-1298.
Foundation item: Project(41312040404) supported by the National Defence “15” Pre-Research Foundation of China
Corresponding author: LI Chang-qing; Tel: +86-451-86402310; E-mail: plumevergreen@163.com


