J. Cent. South Univ. (2016) 23: 1618-1625
DOI: 10.1007/s11771-016-3216-8
Oil spill sorption using raw and acetylated sugarcane bagasse
Reza Behnood1, Bagher Anvaripour1, Nematollah Jaafarzadeh2, 3, Masoome Farasati4, 5
1. HSE Department of Abadan Faculty of Technology, Petroleum University of Technology, Abadan, Iran;
2. Environmental Technologies Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran;
3. School of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran;
4. Department of Irrigation and Water Resources Engineering, Faculty of Agriculture, Razi University, Iran;
5. Faculty of Agriculture, Gonbad-e Kavoos University, Gonbad, Iran
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
In the recent decades oil spills in the aquatic environments are one of the major sources of environmental pollutions, which are steadily growing with the increase in oil consumption. Adsorption is a rapid and cost effective process to minimize the environmental impacts of oil spills and cleanup these pollutants. In this work, the crude oil sorption capacity was examined with raw sugarcane bagasse and acetylated sugarcane bagasse. Results show that the acetylated bagasse was significantly more oleophilic than the raw bagasse and acetylation reaction can increase bagasse oil sorption ability by about 90%. The maximum sorption capacities of acetylated bagasse were obtained about 11.3 g and 9.1 g in dry system (crude oil sorption) and oil layer sorption, respectively. The physicochemical characteristics of the sorbents such as composition, water solubility, moisture content and density were measured according to ASTM standard methods. Also Fourier transform infrared spectroscopy (FTIR) of raw and acetylated bagasse was performed to investigate the effect of acetylation on sugarcane bagasse structure.
Key words:
crude oil; adsorption; natural sorbent; sugarcane bagasse; acetylated bagasse;
1 Introduction
In the recent years water pollution of seas and oceans is a growing concern for several countries contributed to the destruction of biodiversity and the rest of the earth, too. Oil constitutes one of the major sources of contamination in seas and navigable waters. Spilled oil has an undesirable taste and odor that affects tourism and economy. Therefore, spilled oil causes enormous environmental problems unless it is removed as quickly as possible. So, water sources protection must be one of the major topics in our life and necessary actions should be done to remove these pollutants because we depend on water [1-5].
Table 1 lists different technologies for oil spill cleanup methods [6-7]. The best available methods for oil spill cleanup include chemical treatment, gravity separation, parallel-plate coalescers, gas floatation, cyclone separation, granular media filtration, cartridge filtration, adsorption, micro filtration and ultra membranes. Some of these techniques are expensive or time-consuming [8].
Nowadays natural and synthetic sorbents are applied as single solution for oil spills since this technique is effective, rapid and cost saving for cleaning these pollutions and reduce environmental effects [9-11]. Oil sorbents can be divided into three basic categories: inorganic mineral products, organic synthetic products, and organic natural products [12].
In Table 2, natural organic sorbents for different types of oil pollution were reviewed.
In this work the crude oil sorption capacity was examined with raw and acetylated sugarcane bagasse and theirs properties such as composition, water solubility, moisture content and density were measured. Then sorption experiments in batch system were done and effect of contact time, particle size, water salinity and crude oil amounts was studied.
Sugarcane bagasse is an agricultural by-product from the cane sugar refining process. It is the residue fibre remaining when sugarcane is pressed to extract the sugar. Huge amounts (millions of metric tons per year) of this waste are produced worldwide. However, only a small portion of this material is actually reused as fuels in sugar factories or as raw material for pulp and paper products [17].
Table 1 Oil spill cleanup methods [6-7]

Table 2 Natural organic sorbents for crude oil sorption

2 Experimental methods
2.1 Materials
Acetic anhydride, N-bromosuccinimide (NBS), NaCl, MgCl2, Na2SO4 , CaCl2, acetone and ethanol all of analytical grade were obtained from Merck.
Crude oil with a specific gravity of 0.86 at 15 °C was obtained from Abadan’s oil refinery, Iran. Raw sugarcane bagasse was obtained from Karoun Agro- Industry factory, Southwest of Iran. It was washed with tap water and distilled water to remove dust, then dried in open air. After that bagasse was cut into small pieces and sieved with different mesh sizes: 8, 18, 20, 30 and 60.
2.2 Synthesis of acetylated bagasse
Natural sorbents are modified by chemical reactions to increase their sorption capacity. To improve the oleophilic and hydrophobic properties of bagasse for oil spill cleanup, raw bagasse with mesh number 30-60 was treated with acetic anhydride in the presence of NBS (N-bromosuccinimide) as a catalyst, under mild conditions [18].
Raw bagasse was washed with distilled water and oven-dried at 80 °C for 16 h. The amounts of substrate and reactant were combined in a ratio of 1:20 (15 g bagasse and 300 mL acetic anhydride) and the amount of the catalyst was 1% (3 g NBS) as suggested by SUN et al [19] and CHUNG et al [20]. The reactants were put in a 500 mL pyrex batch reactor that was equipped with a reflux condenser. This setup was put in oil bath and heated at 100 °C for 2.5 h that reacted at atmospheric pressure. After reaction time the reactor was removed from the oil bath, and the modified bagasse was thoroughly washed with ethanol and acetone to remove the un-reacted acetic anhydride and acetic acid by- product. The products were then dried in an oven at 80 °C for 16 h.
Raw bagasse is a combination of cellulose, lignin, and hemicellulose. The composition was measured using standard methods in Table 3.
Table 3 Raw sugarcane bagasse composition

2.3 Adsorption experiments
The tests used in this work to determine the crude oil sorption capacity were done in batch system, according to the standard procedures including ASTM F726-99 for sorption experiments and ASTM D1141-98 to produce saline water substitute sea water.
Sorption experiments were done for dry system (crude oil sorption in the absence of water) and crude oil layer system.
For dry system, 50 mL of crude oil was introduced in 250 mL beaker and then 1 g of sorbent was added to crude oil. For crude oil layer sorption, 100 mL of artificial sea water was put in 250 mL beaker. Crude oil was added to form an oil layer with a specified thickness. Then, sorbent was spread over the surface. In the experiments performed to survey the effect of crude oil layer thickness, the amounts of contamination related to the thicknesses of 2.4, 5 and 8 mm were, 7, 15.04 and 24.06 g of crude oil, respectively, and for salinity effect, 3 levels were evaluated, 0, 20 and 40 g/L.
After proper sorption time, sorbent was removed with the net that it was hanged over the beaker for 5 min to provide the falling down of crude oil that was not adsorbed.
The remaining oil was separated from the water and its weight was recorded. Each experiment was performed twice and the sorption capacity was calculated using Eq. (1):
 (1)
(1)
where Q is the oil sorption capacity; m1 is mass of adsorbed oil; m2 is mass of sorbent; Mass of adsorbed oil(g) equals mass of added oil(g) minus mass of remaining oil(g); Mass of remaining oil equals VR(1) plus VR(2); VR(1) is remaining oil visible layer on water surface that was separated by decanter; VR(2) is oil shines on water and eventually dissolved components of oil product in aqueous phase that was determined by COD test.
3 Results and discussion
Figures 1 and 2 show particles which pass through sieves with different mesh sizes.
As seen in Figs. 1 and 2, the particles with different fibre length and equal diameter pass through the sieve due to fibrous form of the bagasse particles. This causes formation of heterogeneous and non-uniform particles in large sieves and the number of larger particles in unit of mass is low due to higher bulk density. For mesh sizes smaller than 1 mm, the effect of decreasing size is higher, because particles are more homogenous in these sizes and the number of particles increases more considerably due to lower bulk density.
Raw bagasse is a combination of cellulose, lignin, and hemicellulose. It is a material that adsorbs hydrophilic and hydrophobic materials.
As can be seen from Table 4, modification decreases bagasse solubility in water. That is may be due to 2 h reaction of modified bagasse in acetic anhydride, because modification process with acetic anhydride places a relatively hydrophobic chain on the raw bagasse structure.

Fig. 1 Raw bagasse through passing sieves with different mesh sizes:

Fig. 2 Raw bagasse and optical microscope image passing through sieves with of 30 mesh (a) and 60 mesh (b, c, d)
Table 4 Data of sorbents solubility in water according to ASTM D5029-98

Table 5 Moisture content in different sorbents

As a result of the acetylation reaction, bagasse gained weight through the substitution of higher- molecular-weight functional groups (acetyl group) for low-molecular-weight functional groups (hydroxyl group). Therefore weight percent gain of sugarcane bagasse due to acetylation was used as a measure of the extent of substitution because this reaction is a single site substitution without polymerization. The weight percent gain was calculated using the following formula [20]:
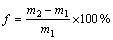 (2)
(2)
where f is percent gain; m2 is mass after reaction minus mass before reaction; m1 is mass before reaction.
The weight percent gains for acetylated bagasse in this work are about 23.4%.
The results show the densities of raw and modified sugarcane bagasse according to ASTM D2854-96, equal to 473 kg/m3 and 367 kg/m3, respectively, which verify the fact that this substance, being placed in the environment, floats on the water after a short period of time.
The identification of the important bands seen in the FTIR diagram (Fig. 3) is according to the previous studies of sugarcane bagasse [17], banana trunk fibers [21] and fatty acid grafted sawdust [15].
In Fig. 3, the strong signal at about 3412 cm-1 is attributed to the stretching vibrations of hydroxyl groups present in cellulose, hemicellulose, and the lignin of sugarcane bagasse. The medium signal, between 1500 cm-1 and 1750 cm-1, is ascribed to carboxylic groups present in lignin and hemicellulose. The bands at 2920.26 cm-1 and 2850 cm-1 correspond to asymmetric C—H and symmetric C—H stretching of CH2-groups respectively. The band at 1248.38 cm-1 is due to C—O stretching in hemicellulose.
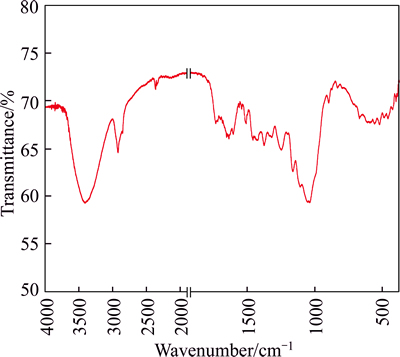
Fig. 3 Infrared spectrum of raw bagasse
Modification process with acetic anhydride places a relatively hydrophobic chain on the raw bagasse structure. Regardless, the chain provides a hydrophobic envelope for the raw bagasse. The lack of water adsorption by the modified bagasse demonstrates this envelope.
The role of NBS is not clear in the acetylation reaction, but a plausible explanation is that NBS might act as a source for Br+, which in turn activates the carbonyl groups of acetic anhydride to produce the highly reactive acylating agent (CH3—CO—N—OCCH2CH2CO—). This acylating agent reacts with hydroxyl groups of raw bagasse, which upon elimination of NBS produces acetylated sugarcane bagasse (SCB— O—CO—CH3). Figure 4 shows the mechanisms of this reaction [7, 19].
Figure 5 illustrates the FT-IR spectrum of acetylated bagasse. Major change before and after acetylation is a reduced peak in the region, 3700-3400 cm-1 for O—H (clearly at 3446 cm-1), indicating that the hydroxyl group contents in modified bagasse were reduced after reaction. The other changes are occurrence of two important ester bands at 1749, and 1244 cm-1, which are attributed to adsorption by carbonyl bonds (C=O ester), and—C—O— stretching in esters (—C—O—in acetyl group), respectively.
These changes in the FT-IR spectra are consistent with those of acetylated cellulosic materials reported by other researchers [19-20]. The results indicate that the acetyl functional group has been successfully attached to bagasse at the expense of the hydroxyl group. The absence of peaks at 1700 cm–1 and 1840-1760 cm–1 in Fig. 5 shows that the by-product (acetic acid) and unreacted reactant (acetic anhydride) are successfully removed during the purification step [19-20].
The effect of contact time on the sorption capacity for dry system is shown in Fig. 6.
It is clear that sorption capacity increases with the contact time in the first 5 min, then sorption reachs equilibrium.
For particle size effect it was seen that decreasing the average particle size (increasing mesh number) increases oil sorption capacity of bagasse, where an optimum is obtained at an average particle size of 0.2 mm. However, decreasing further the average particle size decreases the oil sorption capacity.
Increasing of oil sorption capacity with decreasing particle size can be due to increase in the surface area subjected to the oil bath.
Decreasing of oil sorption capacity with decreasing particle size (smaller than 0.2 mm) can be due to accumulation of small particles on each other which results in plugging pores and capillaries exist between fibres and the reverse decreasing after 200 μm may be due to decreasing of the surface area subjected to oil bath [10, 22].
The experimental observations show that all sorbents float on the surface water after crude oil sorption because they have lower density than water and collect easily after oil sorption.
Figure 7 shows the effect of particles size and sorption time for a crude oil layer on the surface of artificial sea water. 7 g crude oil was added to 150 mL water, forming a layer of about 2.4 mm thickness. Then sorbent (1 g raw -0.5 g modified bagasse) was spread over the surface.
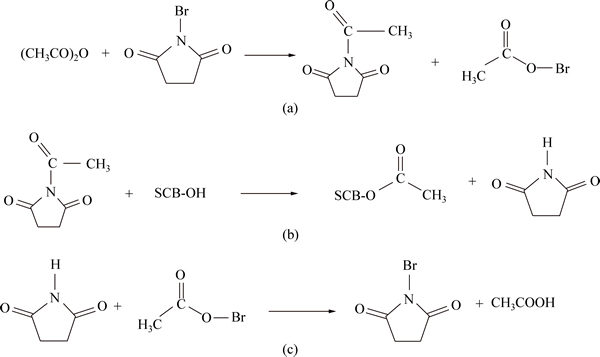
Fig. 4 Mechanisms of acetylation of raw bagasse using NBS as catalyst [16, 19]
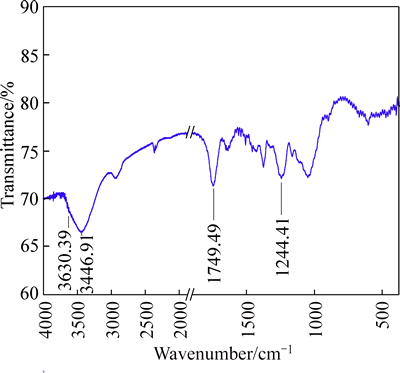
Fig. 5 Infrared spectrum of modified bagasse
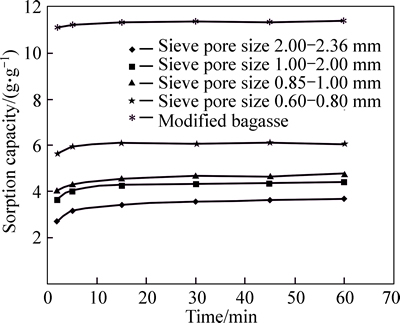
Fig. 6 Time effect for dry system
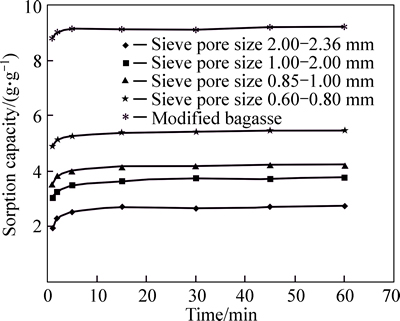
Fig. 7 Effect of sorption time for crude oil layer
Considering the diagram, the maximum sorption capacity has reached after about 5 min.
Removal efficiency of acetylated bagasse was seen about 100 % and as seen in Fig. 8, the raw bagasse meshsize 30 has the removal efficiency 78% for the crude oil layer.
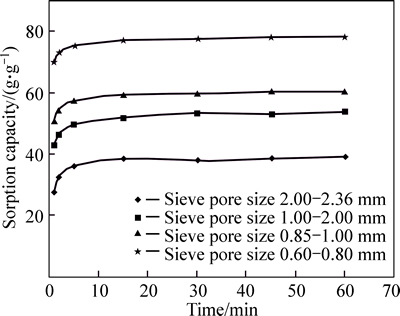
Fig. 8 Crude oil removal efficiency for crude oil layer
The data show that this hydrophobic bagasse would operate effectively as an oil adsorbing material for oil and water mixtures. The raw and modified bagasse would float on the surface of the water, as would the oil. The acetylated bagasse would selectively adsorb the oil and would remain on the surface to be removed when the application was complete. The material containing oil can be used as a fuel in the production of sugarcane or other industrial heating processes. Raw bagasse would adsorb both water and oil from the mixture of these materials. However, raw bagasse would be an effective adsorbent of oil alone from a non-aqueous environment. Additionally, raw bagasse was the most cost effective material in this study.
But the results showed that adsorbed water for modified bagasse is lower than raw bagasse in same particle size, because modification process with acetic anhydride replaces a hydrophobic functional group in the raw bagasse structure and improves hydrophobic and oleophilic properties of bagasse. So, modified sugarcane bagasse adsorbs more crude oil selectively.
Figure 9 shows the effects of crude oil layer thickness and sorbent doses on the oil sorption capacity for raw bagasse. It can be seen that by increasing oil layer thickness, crude oil sorption approaches to the dry system mode,and the sorption capacity is closer to its maximum value in the dry system. In this state, water adsorption reduces, since less sorbents would be in contact with water.
In these experiments, the maximum oil sorption capacity was obtained for 3 g sorbent for 8 mm crude oil layer, about 18 g.
According to the experiments for effect of saline water (Fig. 10), it can be seen that this parameter has not a strong and significant influence on the oil sorption rate. It could be due to the fact that in the adsorption process,the oil layer is formed on the water surface and the sorbent will first adsorb the oil. Thus, it would have less contact with the saline water. But an increase in the water salinity causes a decrease in VR(2) that can be due to decrease in the solubility of the crude oil in the water.
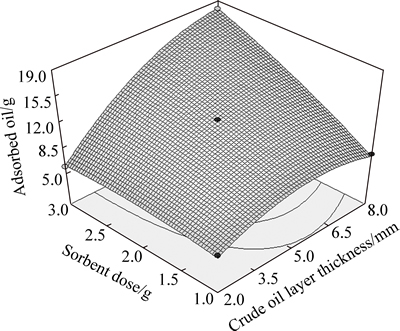
Fig. 9 Effect of sorbent dose and crude oil layer thickness on adsorbed oil
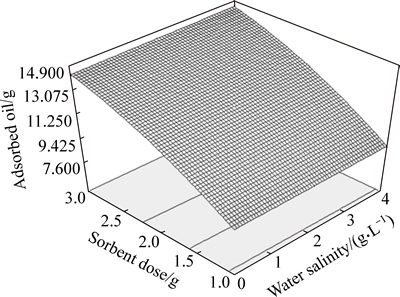
Fig. 10 Effect of salinity and sorbent dose on crude oil layer sorption
In an experiment conducted for sorbents in distilled and saline water of 40 g/L salinity, it was found that the saline water adsorbed is 7%-10%. But as the sorption experiments showed, this phenomenon did not make any significant change in the crude oil layer sorption from the water surface.
4 Conclusions
1) The modification process produced a material significantly more hydrophobic than the raw bagasse. This modified bagasse had little affinity for water and good affinity for oil.
2) The acetylated bagasse would be the most suitable for applications where oil is to be removed from an aqueous environment.
3) Raw bagasse had been shown to adsorb oil better than water. For oil adsorbing applications in the absence of water, the raw bagasse was found to be an excellent material.
4) The sorbent capacity is found to be dependent on the adsorption time and the other conditions such as crude oil layer thickness, sorbent dose and particle size. This sorbents showed a rapid and good oil sorption capacity in different situations.
5) The maximum adsorption capacities of raw and modified bagasse for dry system were obtained about 6 g and 11.3 g respectively, and for 2.4 mm layer thickness seen about 5.4 g and 9.1 g crude oil respectively .
Acknowledgements
The authors would like to thank Karoun Agro- ndustry Factory Staff, Research and Development Office at Abadan’s Oil Refinery and the others that helped us to complete this research.
References
[1] ZHAO J, XIAO C F, XU N K. Diffusion and swelling behavior in treatment of oil spill to semi-interpenetrating polymer network from oil-absorptive fiber [J]. Journal of Dispersion Science and Technology, 2012, 33: 1197-1203.
[2] PARK I, EFIMENKO K, SJ BLOM J, GENZER J. Rapid removal of organics and oil spills from waters using silicone rubber “Sponges” [J]. Journal of Dispersion Science and Technology, 2009, 30: 318-327.
BLOM J, GENZER J. Rapid removal of organics and oil spills from waters using silicone rubber “Sponges” [J]. Journal of Dispersion Science and Technology, 2009, 30: 318-327.
[3] COJOCARU C, MACOVEANU M, CRETESCU I. Peat-based sorbents for the removal of oil spills from water surface: Application of artificial neural network modeling [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2011, 384: 675-688.
[4] ANNUNCIADO T R, SYDENSTRICKER T H D, AMICO S C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills [J]. Marine Pollution Bulletin, 2005, 50: 1340-1346.
[5] KARAN C P, RENGASAMY R S, DAS D. Oil spill clean up by structured fibre assembly [J]. Indian Journal of Fibre & Textile Research, 2011, 36: 190-200.
[6] ZHU X, VENOSA A D, SUIDAN M T, LEE K. Guidelines for the bioremediation of marine shorelines and freshwater wetlands [S]. U.S.EPA, 2001.
[7] DAVE D, GHALY A E. Remediation technologies for marine oil spills: A critical review and comparative analysis [J]. American Journal of Environmental Sciences, 2011, 7: 423-440.
[8] SALEHI K, MOWLA D, KARIMI G H. Removal of oil spills from salty waters by commercial organoclays [J]. Journal of Dispersion Science and Technology, 2012, 33: 1682-1687.
[9] SHE D, SUN R C, JONES G L. Cereal straw as a resource for sustainable biomaterials and biofuels [M]. Elsevier Book Publication, 2010: 209-217.
[10] HUSSEIN M, AMER A A, EL-MAGHRABY A, TAHA N A. Availability of barley straw application on oil spill clean up [J]. International Journal of Environmental Science and Technology, 2009, 6: 123-130.
[11] ROSS S L. Selection criteria and laboratory evaluation of oil spill sorbents (report): update IV. Environmental Canada Protection Services [S]. 1991.
[12] KESHAWY M, EL-HAMOULY S H, ABDUL-RAHEIM A R M, KABEL KH I, ABDEL-MOGHNEY T. Synthesis of oil spill sorbents based on cellulose derivatives [J]. Journal of Dispersion Science and Technology, 2013, 34: 1507-1516.
[13] ADEBAJIO M O, FROST R L, KLOPROGGE J T, CARMODY O, KOKOT S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties [J]. Journal of Porous Materials, 2003, 10: 159-170.
[14] VLAEV L, PETKOV P, DIMITROV A, GENIEVA S. Cleanup of water polluted with crude oil or diesel fuel using rice husks ash [J]. Journal of the Taiwan Institute of Chemical Engineers, 2011, 42: 959-964.
[15] BANERJEE S S, JOSHI, M V, JAYARAM R V. Treatment of oil spill by sorption technique using fatty acid grafted sawdust [J]. Chemosphere, 2006, 64: 1026-1031.
[16] SAYED S A, SAYED A S E, ZAYED A M. Oil spill pollution treatment by sorption on natural Cynanchum Acutum L. Plant [J]. Journal of Applied Sciences and Environmental Management, 2003, 7: 63-73.
[17]  P C, SOUZA T C, FERREIRA C A, HORI C E, ROMANIELO L L. Removal of petroleum hydrocarbons from aqueous solution using sugarcane bagasse as adsorbent [J]. Journal of Hazardous Materials, 2010, 175: 1106-1112.
P C, SOUZA T C, FERREIRA C A, HORI C E, ROMANIELO L L. Removal of petroleum hydrocarbons from aqueous solution using sugarcane bagasse as adsorbent [J]. Journal of Hazardous Materials, 2010, 175: 1106-1112.
[18] SAID A E A, LUDWICK A G, AGLAN H A. Usefulness of raw bagasse for oil absorption: A comparison of raw and acylated bagasse and their components [J]. Bioresource Technology, 2009, 100: 2219-2222.
[19] SUN X F, SUN R C, SUN J X. A convenient acetylation of sugarcane bagasse using NBS as a catalyst for the preparation of oil sorption-active materials [J]. Journal of Materials Science, 2003, 38: 3915-3923.
[20] CHUNG S, SUIDAN M T, VENOSA A D. Partially acetylated sugarcane bagasse for wicking oil from contaminated wetlands [J]. Chemical Engineering Technology, 2011, 34: 1989–1996.
[21] SATHASIVAM K, HARIS M R H M. Adsorption kinetics and capacity of fatty acid-modified banana trunk fibers for oil in water [J]. Water, Air, & Soil Pollution, 2010, 213: 413-423.
[22] HUSSEIN M, AMER A A, SAWSAN I I. Oil spill sorption using carbonized pith bagasse 1. Preparation and characterization of carbonized pith bagasse [J]. Global NEST Journal, 2009, 11: 440-448.
(Edited by YANG Hua)
Received date: 2015-06-02; Accepted date: 2015-10-16
Corresponding author: Nematollah Jaafarzadeh, Professor, PhD; Tel: +989163184501; Fax: +986133388425; E-mail: jaafarzadeh-n@ajums.ac.ir
Abstract: In the recent decades oil spills in the aquatic environments are one of the major sources of environmental pollutions, which are steadily growing with the increase in oil consumption. Adsorption is a rapid and cost effective process to minimize the environmental impacts of oil spills and cleanup these pollutants. In this work, the crude oil sorption capacity was examined with raw sugarcane bagasse and acetylated sugarcane bagasse. Results show that the acetylated bagasse was significantly more oleophilic than the raw bagasse and acetylation reaction can increase bagasse oil sorption ability by about 90%. The maximum sorption capacities of acetylated bagasse were obtained about 11.3 g and 9.1 g in dry system (crude oil sorption) and oil layer sorption, respectively. The physicochemical characteristics of the sorbents such as composition, water solubility, moisture content and density were measured according to ASTM standard methods. Also Fourier transform infrared spectroscopy (FTIR) of raw and acetylated bagasse was performed to investigate the effect of acetylation on sugarcane bagasse structure.


