
Influence of Ti4+ doping on electrochemical properties of
LiFePO4/C cathode material for lithium-ion batteries
HU Guo-rong(胡国荣), GAO Xu-guang(高旭光), PENG Zhong-dong(彭忠东),
DU Ke(杜 柯), TAN Xian-yan(谭显艳), Liu Yan-jun(刘艳君)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 13 November 2006; accepted 12 December 2006
Abstract:
To improve the performance of LiFePO4, single phase Li1-4xTixFePO4/C (x=0, 0.005, 0.010, 0.015) cathodes were synthesized by solid-state method. A certain content of glucose was used as carbon precursor and content of carbon in every final product was about 3.5%. The samples were characterized by X-ray diffraction(XRD), scanning electron microscopy observations(SEM), charge/discharge test, carbon analysis and electrochemical impedance spectroscopy(EIS). The results indicate that the prepared samples have ordered olivine structure and doping of the low concentration Ti4+ does not affect the structure of the samples. The electrochemical capabilities evaluated by charge-discharge test show that the sample with 1% Ti4+ (molar fraction) has good electrochemical performance delivering about an initial specific capacity of 146.7 mA?h/g at 0.3C rate. Electrochemical impedance spectroscopy measurement results show that the charge transfer resistance of the sample could be decreased greatly by doping an appropriate amount Ti4+.
Key words:
Li-ion battery; cathode material; doping; LiFePO4; Ti4+;
1 Introduction
Among cathode materials of Li-ion batteries, such as LiCoO2, LiMn2O4 and LiFePO4, LiFePO4 of the phospho-olivine family reported by PADHI et al[1] has attracted particular attention due to the high energy density, low cost and good environmental compatibility of its basic constituents[2-4]. However, its weakness of diffusion limitation and poor electrical conductivity lead to poor electrochemical performance and makes it difficult to be used as the cathode material for the commercial lithium ion battery. In recent years, many researchers have improved the electrochemical performance by coating carbon[5-8] or preparing suitable procedures to minimize the particle of the sample[9-12] or doping metal ions supervalent to Li+ sites[13] or Fe sites[14-15]. However, the method by coating carbon could not improve the intrinsic electrical conductivity on crystal lattice level. Meanwhile the routes by doping metal supervalent ions could not completely avoid the overgrowth of single crystal during the calcining process and the larger crystal will lead to poor electrochemical performance for the diffusion limitation. To overcome the weakness, Li1-4xTixFePO4/C composites were synthesized by the methods of lattice doping and surface coating in this paper.
2 Experimental
Li1-4xTixFePO4/C (x=0, 0.005, 0.010, 0.015) composites were prepared by solid-state method with stoichiometric Li2CO3 (99.5%), nano-size TiO2 (99.8%), FeC2O4?2H2O (99.0%), NH4H2PO4 (98.0%) and glucose (98.0%) as raw materials. The mixture of the raw materials above were mixed by ball-milling (500 r/min) in alcohol for 2.5 h with agate balls, followed by drying. Then these mixtures were ground with a mortar and pestled, and calcined at 350 ℃ for 8 h in Ar flowing (99.99%). Final firing for crystallization of the olivine phase was made at 750 ℃ for 12 h in Ar flowing ambience. The final product was obtained by further treatments of milling and sieving.
The phase analysis and cell parameters of all samples were determined by using X-ray diffraction (XRD, D/max-r A type Cu Kα1, 40 kV, 300 mA, 10?-70?, Japan). Scanning electron microscope(SEM) was used to examine the microstructures of the cathode material (SEM, JEOL JSM-6360LV). The concentration of carbon in the composite was analyzed by using CS-444 carbon/sulfur determinator (LECO Co. USA).
The electrochemical characterization of the cathode powders was evaluated at room temperature (23 ℃). Cathode electrodes for electrochemical testing were prepared by slurrying Li1-4xTixFePO4/C powder (80%, mass fraction) with 10% (mass fraction) acetylene black (AB) and 10% (mass fraction) polyvinylidene difluoride (PVDF, in N-methyl pyrolidinone(NMP), and then this mixture was cast onto a aluminum foil. After vacuum drying at 120 ℃ for 8 h, the electrode disks with 12 mm in diameter were punched and weighed. The cathodes were incorporated into laboratory-scale 2025 coin-type cells. A micro-porous polypropylene film (Celgard2400) was used as a separator and 1 mol/L LiPF6 solution with 1?1 of volume ratio (EC+DEC) was used as electrolyte solution. Lithium metal foil was used as counter electrode. All cells were assembled inside a glove box filled with ultra-pure argon. Charge/discharge characteristics of the active material were recorded at 0.3C (51 mA/g) rate over a voltage range of 2.5-4.1 V by using a battery test system (LAND CT2001A).
The samples were applied to study the electrochemical impedance spectroscopy respectively by using Model 273A Potentiostat/Galvanostanostat and Model 5210 Dual Phase Lock-in Amplifier, USA. The active material electrodes were used as working electrodes. Lithium metal foil was used as the counter electrode and reference electrode and 1 mol/L LiPF6/EC+DMC (1?1 in volume ratio) was used as the electrolyte solution.
3 Results and discussion
Fig.1 shows XRD patterns of Li1-4xTixFePO4/C (x=0, 0.005, 0.010, 0.015) samples. The crystal phases of all the samples are to be an ordered olivine structure indexed by orthorhombic Pnma, and no other impurities are detected. It is obvious that doping a low amount of Ti4+ does not affect the structure of the samples.
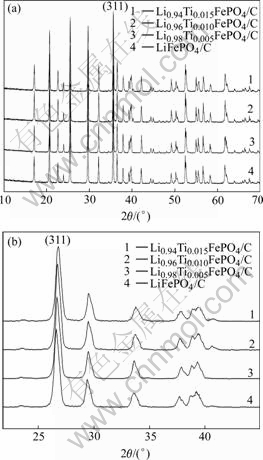
Fig.1 XRD patterns of Li1-4xTixFePO4/C
Doping Ti4+ would be inclined to occupy Li+ sites because the cation radii of lithium (r(Li+)=68 pm) is close to that of titanium (r(Ti4+)=64 pm). Besides, Li1-nxxMen+FePO4 is a kind of good P-type semiconductor material with relative high electric and ionic conductivity[13]. Table 1 shows the cell parameters of the samples calculated by XRD analysis. From the Table 1 we can know that the lattice parameter changes slightly with the increase of the amount of Ti4+, while the lattice parameters b and c reduce gradually. Meanwhile it is clearly to find the phenomena from the Fig.1 that the diffraction peaks (311) of samples shift right gradually when more Ti4+ ions are doped, which means the unit cell volume minimizes gradually according to the Prague equation. The volume contraction of the doped samples might be due to the coexistence of lithium ion vacancy caused by Ti4+ dopant and the strong bond energy between Ti4+ cation and oxygen atom.
Table 1 Cell parameters of Li1-4xTixFePO4/C
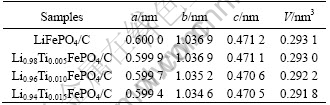
Fig.2 shows SEM images of LiFePO4/C (Fig.2(a)) and Li0.96Ti0.01FePO4/C (Fig.2(b)). Scanning electron microscopy(SEM) analysis result indicates that the surface of the particle turns sleeker and it is less granular and displays similar spheric particle with a good dispersion by further treating the products. Besides, the distribution of particle size of the samples is uniform. It is obvious that these factors are helpful to increase the tap density of the powder material. While most samples reported by other authors always consisted of subgrain, and the particle size of single grain was less than 0.5 μm[2,5]. Obviously, their samples required to coat more amount of carbon, so the content of active material has to be reduced. On the other hand, coating more carbon would also lead to the low tap density. Furthermore, the size of single grain has a great effect on the electrochemical performance of LiFePO4 for the diffusion mechanism of lithium ion and electron through the interface of LiFePO4/FePO4 in the electrode process. Thus, for the pure LiFePO4 samples, the reversible capacity would be decreased rapidly with the increase of single crystal size.

Fig.2 SEM images of LiFePO4/C (a) and Li0.96Ti0.01Fe-PO4/C (b)
Fig.3(a) shows voltage profiles of Li1-4xTixFePO4/C electrodes in the first cycle. The cells were cycled between 2.5 and 4.1 V at a current density of 0.3C (51 mA/g) rate. Typical two-phase reactions between LiFePO4 and FePO4 with a 3.45 V (vs Li/Li+) plateau are observed in all samples. The discharge capacities of LiFePO4/C cathode, Li0.98Ti0.005FePO4/C cathode, Li0.96Ti0.01FePO4/C cathode, and Li0.94Ti0.015Fe- PO4/C cathode show the initial discharge capacity of 128.4, 139.5, 146.7 and 99.5mA?h/g respectively. Because all samples were prepared under same technical conditions except doping different content of Ti4+, the effect of doping Ti4+ on the electrochemical performance could be confirmed. It is not a contradictory that the specific capacity of the undoped sample prepared by us was lower than those of others, which can be illustrated by SEM image. Fig.3 (b) shows the cycle performance of all samples cycled for 20 times. All samples have good cycle performance because of the stable structure in the process of charging and discharging[16].
Fig.3 Charge/discharge curves of Li1-4xTixFePO4/C electrodes (a) and cycle performance of Li1-4xTixFePO4/C electrodes (b)
The electrochemical performance of the doped samples is better than that of undoped one when the doping amount of Ti4+ was less than 1.5%. More amount of Ti4+ dopant on Li+ sites may block the diffusive path of lithium ion because the diffusion coefficient of Li+ is fast only along the tunnel c-axis[17]. The sample doped by appropriate amount of Ti4+ can get better electrochemical properties, which could be interpreted as the enhancement of electronic conductivity by ion doping. According to the mechanism proposed by CHUNG et al[13], the ion dopant mainly takes the site of Li+, lead to the coexistence of Fe2+ and Fe3+ in single phase, and improving crystal electronic conductivity apparently.
Moreover, the amount of carbon in these compounds is about 3.5% (mass fraction) evaluated by using CS-444 carbon/sulfur determinator (LECO Co. USA), which is less than others reported in Refs.[5-6]. Meanwhile, glucose can dissolve in alcohol distributed uniformly in the precursor of cathode material and is a kind of simple and cheap raw material. Besides, carbon can be used as an effective reducing agent and avoid the oxidation of ferrous phase during the calcining process. After calcining in Ar flowing ambience, the refined carbon would deposit on the surface of crystal particle uniformly, increase the electronic conductivity and improve the interface of charge transport when used as cathode material[18].
In order to testify the electrochemical property of the sample Li0.96Ti0.01FePO4/C, the coin 2025 type battery was made with 96.0% Li0.96Ti0.01FePO4/C powder (mass fraction) and 4.0% PVDF (mass fraction), and was used as cathode, and lithium foil was used as counterpart. The charge–discharge behaviors are compared in Fig.4.
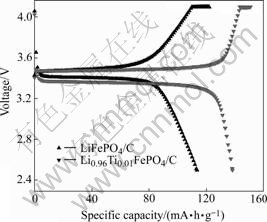
Fig.4 Charge/discharge behaviors of LiFePO4/C and Li0.96Ti0.01FePO4/C (mass ratio of active material to PVDF is 96?4)
From Fig.4 we can clearly know that the sample Li0.96Ti0.01FePO4/C has a good electrochemical performance delivering about 138.5 mA?h/g without additional electric additives. Meanwhile LiFePO4/C only can keep discharge specific capacity of 115.3 mA?h/g.
Fig.5 represents the Nyquist plots of Li1-4xTixFePO4/C samples at an ambient temperature. In Fig.5, the impedance spectra are combinations of a depressed semicircle in high frequency region and a straight line in low frequency region. Interpretation of the impedance spectra was based on the equivalent circuit in Fig.5(b). The symbols Rs, Rct, Cd, and Zw, denote the solution resistance, charge-transfer resistance, capacitance of the double layer, and Warburg impedance, respectively. In the high-frequency region, the intercepts with real impedance Zre axis of Li0.96Ti0.01FePO4/C and LiFePO4/C composite are 125 and 332 Ω, respectively. This value is believed to be the total electric resistance of the electrode materials, electrolyte resistance and electric leads[19]. The decrease of the total electric resistance could be attributed to doping Ti4+. Therefore, the total electric resistance of the composite cathode is reduced by doping of Ti4+ with a certain content, indicating that doing metal ion is an effective way to improve the electrochemical activity of LiFePO4/C.
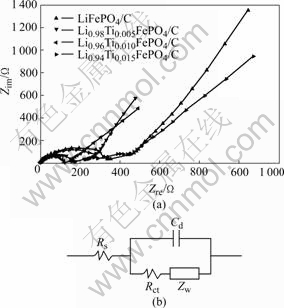
Fig.5 EIS spectra of Li1-4xTixFePO4/C cathodes at 25 ℃ (a) and equivalent circuit (b)
4 Conclusions
1) Single phase Li1-4xTixFePO4/C composite was successfully synthesized by solid-state reaction.
2) The performance of electron conductivity and ion transport could be improved by using nano-sized TiO2 and glucose as precursor both doping and coating. Doping a appropriate amount of Ti4+ can improve the electrochemical performance of lithium iron phosphate.
3) The sample of Li0.96Ti0.01PO4/C shows excellent electrochemical performance and could reach a specific capacity of 138.5 mA?h/g without additional electric additives.
References
[1] PADHI A K, Najundaswamy K S, Goodenough J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144: 1188-1194.
[2] Bauer E M, Bellitto C, Pasquali M, Prosini P P, Righini G. Versatile synthesis of carbon-rich LiFePO4 enhancing its electrochemical properties [J]. Electrochem Solid-State Lett, 2004, 7(4): A85-A90.
[3] Wang G X, Bewlay S, YAO J, AHN J H, DOU S X, LIU H K. Characterization of LiMxFe1-xPO4 (M=Mg, Zr, Ti) cathode materials prepared by the sol-gel method [J]. Electrochem Solid-State Lett, 2004, 7(12) A503-A509.
[4] Huang H, Yin S C, Nazar L F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates [J]. Electrochem Solid- State Lett, 2001, 4: A170-A172.
[5] HU G R, GAO X G, PENG Z D, CHEN Z Y, TAN X Y, YU X Y. Synthesis of LiFePO4/C composite electrode with enhanced electrochemical performance [J]. Trans Nonferrous Met Soc China, 2005, 15(4): 795-798.
[6] Myung S T, Komaba S, Hirosaki N, Yashiro H, Kumagai N. Emulsion drying synthesis of olivine LiFePO4/C composite and its electrochemical properties as lithium intercalation material [J]. Electrochimica Acta, 2004, 49(24): 4213-4222.
[7] Baker J, Saidi M Y, Swoyer J. L. Lithium iron(Ⅱ) phospho-olivines prepared by a novel carbonthermal reduction methods [J]. Electrochem Solid-State Lett, 2003, 6(3): A53-A55.
[8] ZHANG Bao, LI Xin-hai, ZHU Bing-quan, WANG Zhi-xing, GUO Hua-jun. Low temperatures synthesis and electrochemical properties of LiFePO4/C cathode [J]. Journal of Central South University: Science and Technology, 2006, 37(3): 505-508. (in Chinese)
[9] YANG S F, Zavalij P Y, Whittinggham M S. Hydrothermal synthesis of lithium iron phosphate cathodes [J]. Electrochem Commun, 2001, 3(9): 505-508.
[10] Park K S, Kang K T, Lee S B, Kim G Y, Park Y J, Kim H G. Synthesis of LiFePO4 with fine particle by co-precipitation method [J]. Materials Research Bulletin, 2004, 39(12): 1803-1810.
[11] Yamada A, Chung S C, Hinokuma K. Optimized LiFePO4 for lithium battery cathodes [J]. J Electrochem Soc, 2001, 148(3): A224-A229.
[12] Cho T H, Chung H. Synthesis of olivine-type LiFePO4 by emulsion-drying method [J]. J Power Sources, 2004, 133(2): 272-276.
[13] Chung S Y, Blocking J T, Chiang Y M. Electronically conductive phospho-olivines as lithium storage electrodes [J]. Nature Mater, 2002, 2: 123-128.
[14] Mi C H, Zhang X G, Zhao X B, Li H L. Synthesis and performance of LiMn0.6Fe0.4PO4/nano-carbon webs composite cathode [J]. Materials Science & Engineering, 2006, 129: 8-13.
[15] Li G H, Kudo Y, Liu KY, Azuma H, Tohda M. X-ray absorption study of LixMnyFe1-yPO4 [J]. J Electrochem Soc, 2002, 149(11): A1414-A1418.
[16] Kim H S, Cho B W, Cho W. Cycling performance of LiFePO4 cathode material for lithium secondary batteries [J]. J Power Sources, 2004, 132(1/2): 235-239.
[17] Venkat S, John N. Existence of path-dependence in the LiFePO4 electrode [J]. Electrochem Solid-State Lett, 2006, 9(3): A110-A114.
[18] Chung H T, Jang S K, Ryub H W, Shimc K B. Effects of nano-carbon webs on the electrochemical properties in LiFePO4/C composite [J]. Solid-State Commun, 2004, 131(8): 549-554.
[19] Bard A J, Faulkner L R. Electrochem Methods: Fundamental and Applications [M]. New York: Wiley, 1980.
Foundation item: Project(04JJ0388) supported by the National Science Foundation of Hunan Province, China
Corresponding author: GAO Xu-guang; Tel: +86-731-8830474; E-mail: csugaoshou@hotmail.com
Abstract: To improve the performance of LiFePO4, single phase Li1-4xTixFePO4/C (x=0, 0.005, 0.010, 0.015) cathodes were synthesized by solid-state method. A certain content of glucose was used as carbon precursor and content of carbon in every final product was about 3.5%. The samples were characterized by X-ray diffraction(XRD), scanning electron microscopy observations(SEM), charge/discharge test, carbon analysis and electrochemical impedance spectroscopy(EIS). The results indicate that the prepared samples have ordered olivine structure and doping of the low concentration Ti4+ does not affect the structure of the samples. The electrochemical capabilities evaluated by charge-discharge test show that the sample with 1% Ti4+ (molar fraction) has good electrochemical performance delivering about an initial specific capacity of 146.7 mA?h/g at 0.3C rate. Electrochemical impedance spectroscopy measurement results show that the charge transfer resistance of the sample could be decreased greatly by doping an appropriate amount Ti4+.
Versatile synthesis of carbon-rich LiFePO4 enhancing its electrochemical properties [J]. Electrochem Solid-State Lett, 2004, 7(4): A85-A90." target="blank">[2] Bauer E M, Bellitto C, Pasquali M, Prosini P P, Righini G. Versatile synthesis of carbon-rich LiFePO4 enhancing its electrochemical properties [J]. Electrochem Solid-State Lett, 2004, 7(4): A85-A90.


