
Simultaneous removal of Cr(Ⅵ) and phenol in consortium culture of Bacillus sp. and Pseudomonas putida Migula (CCTCC AB92019)
LIU Yun-guo(刘云国), PAN Cui(潘 翠), XIA Wen-bin(夏文斌), ZENG Guang-ming(曾光明),
ZHOU Ming(周 鸣), LIU Yuan-yuan(刘媛媛), KE Jie (柯 杰), HUANG Chao(黄 超)
College of Environmental Science and Engineering, Hunan University, Changsha 410082, China
Received 19 November 2007; accepted 8 April 2008
Abstract:
The simultaneous removal of Cr(Ⅵ) and phenol in a consortium culture containing Cr(Ⅵ) reducer, Bacillus sp. and phenol degrader, Pseudomonas putida Migula (CCTCC AB92019) was studied. Phenol was used as the sole carbon source. Bacillus sp. utilized metabolites formed from phenol degradation as electron donors and energy source for Cr(Ⅵ) reduction. Optimum Cr(Ⅵ) reduction was observed at a phenol concentration of 150 mg/L and an initial Cr(Ⅵ) concentration of 15 mg/L. Both the Cr(Ⅵ) reduction and phenol degradation were influenced by the cell composition of the culture, but the phenol degradation was not significantly affected by the content of Bacillus sp. The experiments also showed that the amount of phenol degraded was more than that stoichiometrically required for Cr(Ⅵ) reduction.
Key words:
phenol; chromate; consortium culture; biodegradation;
1 Introduction
Hexavalent chromium has been recognized as one of the most popular environmental pollutants due to its widespread use in modern industries such as textile manufacture, iron and steel industries, ore processing, and leather tanning[1-2]. Both trivalent chromium and hexavalent chromium exist in wastewaters. Cr(Ⅵ) is classified by the US EPA as a group A carcinogen based on its chronic and subchronic effects, while the trivalent chromium Cr(Ⅲ), on the other hand, is less toxic and less soluble than Cr(Ⅵ), and even essential to human health in trace amount[3-5]. Thus, the transformation of Cr(Ⅵ) to the more immobile and less toxic Cr(Ⅲ) represents a detoxification process and an attractive and useful process has been developed for the remediation of Cr(Ⅵ) pollution. Cr(Ⅵ) could be reduced into Cr(Ⅲ) by biological and chemical methods. Biological treatment of Cr(Ⅵ) is effective and can significantly reduce the quantity of the produced sludge, therefore becomes a more preferable process in Cr(Ⅵ) treatment[6-7]. Many species of microorganisms, including strains of Escherichia[8-9], Bacillus[3, 10], Shewanella[11-12], Pseudomonas[13-14] have been identified and used for the degradation of Cr(Ⅵ) under either aerobic or anaerobic conditions due to their high removal efficiency.
In the world, organic pollutants including phenol, naphthalene and trichloroethylene have been found at high concentrations in Cr(Ⅵ) polluted wastewaters discharged from wood preserving plants, petroleum refining plants, leather tanning plants and metal finishing plants[15-16]. Thus, microbial reduction of Cr(Ⅵ) with simultaneous degradation of aromatic pollutants has received much concerns.
The known electron donors for Cr(Ⅵ) reduction are generally limited to amino acids and aliphatic compounds. The use of aromatic compounds as electron donor and carbon source for microbial reduction of Cr(Ⅵ) represents the potential for simultaneous detoxi- fication of organic compounds since both Cr(Ⅵ) and organic compounds are often found together in polluted sites. KUO and GENTHNER[17] found that the presence of heavy metals can affect the outcome of anaerobic bioremediation of aromatic pollutants. SHEN et al[18] also investigated the feasibility of simultaneously removed Cr(Ⅵ) and benzoate, and received good results at last. Mixed cultures containing organic compound degraders and heavy metal degraders have been shown to out-perform in both heavy metal and toxic aromatic compound removal.
Bacterial can utilize a number of organic pollutants including phenol, 2-chlorophenol and benzene as the electron donors for Cr(Ⅵ) reduction[19]. In this work, phenol was chosen as aromatic copollutant of Cr(Ⅵ) due to its relatively wide occurrence in Cr(Ⅵ) contaminated sites. The feasibility of simultaneous removal of Cr(Ⅵ) and phenol in a batch coculture consisting of Bacillus sp. and Pseudomonas putida Migula (CCTCC AB92019) was examined.
2 Experimental
2.1 Bacterial strains and medium
The microorganism Pseudomonas putida Migula (CCTCC AB92019) was purchased from China Center for Type Culture Collection, and was stored at 4 ℃ in a nutrient agar medium containing beef extract 3.0 g/L, peptone 3.0 g/L, agar 20.0 g/L and NaCl 5.0 g/L. The pH of the medium was 7.0. The Cr(Ⅵ)-reducer was previously isolated from the chromium landfill, located at a chromate factory in Changsha, China, and was identified as Bacillus sp. The components of the mineral liquid medium were KH2PO4 0.5 g/L, K2HPO4 0.5 g/L, MgSO4·7H2O 0.2 g/L, CaCl2 0.1 g/L, NaCl 0.2 g/L, MnSO4·H2O 0.01 g/L, NH4NO3 1.0 g/L, and the sole carbon source phenol was added to the required concentration. All chemicals used for the culture medium were of analytical grade and in all experiments the working volume was 100 mL.
2.2 Free suspension cultivation
The Bacillus sp. was enriched in a liquid nutrient medium which contained the same components described above in nutrient agar medium except agar, whereas P. putida Migula was enriched in mineral liquid medium with phenol as the sole carbon source. Both bacteria were incubated in the flasks at 150 r/min and 30 ℃ for 12 h. The cultivated cells were harvested by centrifugation at 3 600 r/min for 15 min and then washed with sterile water and re-centrifuged twice. After centrifuging, the active cells were re-suspended in mineral liquid medium.
2.3 Cr(Ⅵ) reduction and phenol degradation experiments
The 250 mL flasks containing 100 mL mineral liquid medium, desired phenol concentration and chromate (K2CrO4) were inoculated with 1 mL cell suspension, then incubated in a shaker controlled at 150 r/min and 30 ℃. All media (except phenol) were autoclaved at 120 ℃ for 20 min before being used in the experiments.
2.4 Analytical methods
Samples (2 mL) were withdrawn at regular time and centrifuged at 10 000 r/min for 15 min to remove the biomass from the liquid phase. Clear supernatants were analyzed for phenol degradation and Cr(Ⅵ) reduction. Hexavalent chromium was determined colorimetrically by using a spectrophotometer (UV 754N) at 540 nm by reaction with diphenylcarbazide in acid solution. Phenol was determined by HPLC (AGILENT 1100), using a Phenomenex C18 column (particle size 5 μm). The eluent was methanol-water mixture (60?40 (v/v)) pumped at a flow rate of 1.0 mL/min. The sample was subject to filtration through a Millipore filter (0.2 μm) before HPLC analysis. The cell wet mass of each bacterium was used to measure the cell composition.
3 Results and discussion
3.1 Simultaneous Cr(Ⅵ) reduction and phenol degra- dation
The simultaneous removal of phenol and Cr(Ⅵ) in seven different cultures were investigated and the results are listed in Table 1. It can be easily seen that the Cr(Ⅵ) reduction occurred in the consortium with concomitant phenol degradation. The concentration of Cr(Ⅵ) decreased from 20 mg/L to 4.1 mg/L after 60 h of incubation with 100 mg/L phenol as the sole added carbon source (Test 1). Little or no Cr(Ⅵ) reduction and phenol degradation were observed in the autoclaved culture and in the culture without inoculating the bacteria (Tests 6 and 7). This indicated that Cr(Ⅵ) reduction and phenol degradation were accomplished through biological activity. Cr(Ⅵ) reduction was insignificant in the absence of Cr(Ⅵ)-reducing Bacillus sp, while phenol can be degraded by P. putida Migula in the same culture (Test 2). But in the culture without P. putida Migula, both Cr(Ⅵ) reduction and phenol degradation were not evident (Test 4). The concentration of Cr(Ⅵ) reduced from 20 mg/L to 3.84 mg/L when glucose was added in the culture without P. putida Migula, while the concentration of phenol remained nearly the same as beginning (Test 3). More reduction of Cr(Ⅵ) can be observed in the culture with adding phenol than in the culture without adding phenol, indicating that phenol degradation enhanced Cr(Ⅵ) reduction (Tests 1 and 5). Limited Cr(Ⅵ) reduction may be attributed to endogenous respiration of Bacillus sp. By comparing all the data of test, it can be found that Cr(Ⅵ) reduction was correlated to phenol degradation. Phenol was an indispensable energy source in the culture because Cr(Ⅵ) reduction cannot continue after phenol was removed from the culture. The fact that Cr(Ⅵ) reduction was not significant without the phenol degradation indicated that Bacillus sp. cannot use phenol as carbon source directly. So, it can be concluded that the metabolites formed from phenol degradation were used by Bacillus sp. for Cr(Ⅵ) reduction. Phenol degradation probably provided electron donors and energy source for Cr(Ⅵ) reduction. In order to understand the relationship between phenol degradation and Cr(Ⅵ) reduction, more experiments were carried out under different Cr(Ⅵ) concentration, phenol concentration and cell composition.
Table 1 Cr(Ⅵ) reduction and phenol degradation in different cultures
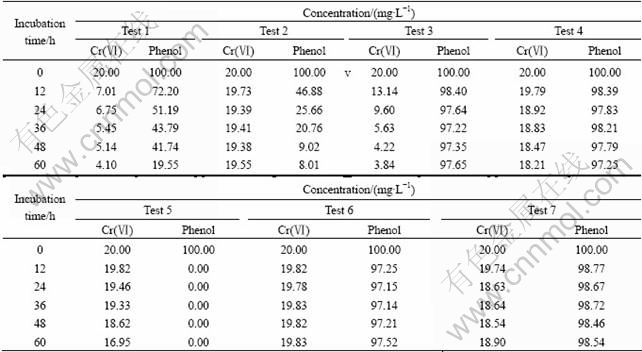
Test1: Culture with all of factors except glucose; Test 2: Culture without Bacillus sp; Test 3: Culture with adding glucose, but no P. putida Migula; Test 4: Culture without P. putida Migula; Test 5: Culture without phenol; Test 6: Autoclaved culture without bacterial; Test 7: Culture with killed cells
3.2 Effect of Cr(Ⅵ) concentration
The phenol concentration and Cr(Ⅵ) concentration at different initial Cr(Ⅵ) concentrations as a function of time can be observed in Fig.1. It can be seen from Fig.1(a) that the average rate of Cr(Ⅵ) reduction increased with initial Cr(Ⅵ) concentration until an optimum Cr(Ⅵ) concentration of 15 mg/L was reached (from 0.08 mg/(L?h) at the initial Cr(Ⅵ) concentration of 5 mg/L to 0.199 mg/(L?h) at 15 mg/L). The culture exhibited a better performance on Cr(Ⅵ) reduction if the initial Cr(Ⅵ) concentration was lower than 15 mg/L. This result may be due to the toxicity effect of chromate on inhibiting the biological activity in the culture. High concentration of Cr(Ⅵ) affected cell growth and then the cells failed to grow and reduce Cr(Ⅵ). The inhibitory effect of Cr(Ⅵ) on bacterial metabolism has been reported in both pure and mixed cultures[20-21].
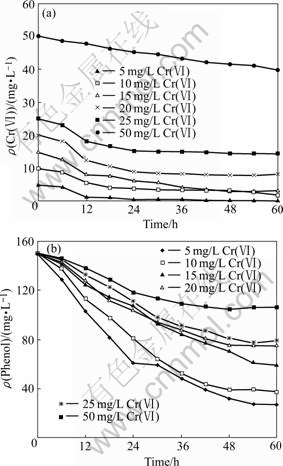
Fig.1 Effect of initial Cr(Ⅵ) concentration on Cr(Ⅵ) reduction (a) and phenol degradation (b) (Initial phenol concentration 150 mg/L and initial Cr(Ⅵ) concentration 5-50 mg/L)
It can be found from Fig.1(b) that the average rates of phenol degradation were 2.06, 1.89, 1.52, 1.26, 1.19 and 0.74 mg/(L?h) at initial Cr(Ⅵ) concentration of 5, 10, 15, 20, 25 and 50 mg/L, respectively. The average degradation rate of phenol declined with increasing the initial Cr(Ⅵ) concentration. This indicated the toxicity and inhibiting effect of Cr(Ⅵ) on phenol degradation. The inhibiting effect of Cr(Ⅵ) on phenol degradation increased with the increase in Cr(Ⅵ) concentration. However, the rate of Cr(Ⅵ) reduction increased with an increase in initial Cr(Ⅵ) concentration from 5 to 15 mg/L, and it reached a maximum value at the initial Cr(Ⅵ) concentration of 15 mg/L before the rate started to decrease. High Cr(Ⅵ) concentration had inhibiting effect on phenol degradation. This effect not only led to significant reduction of the phenol degradation, but also limited Cr(Ⅵ) reduction because of insufficient electron donors produced by phenol degradation. KUO and GENTHNER[17] had investigated the effects of adding some metal ions on biotransformation and biodegradation of 2-Chlorophenol and 3-Chlorobenzoate, and found that the presence of heavy metals could affect the outcome of bioremediation of aromatic compounds.
3.3 Effect of phenol concentration
The effect of phenol concentration on the Cr(Ⅵ) reduction and phenol degradation was investigated over a range of about 25-250 mg/L under an initial Cr(Ⅵ) concentration of 15 mg/L. As shown in Fig.2(a), Cr(Ⅵ) reduction occurred in all cultures with phenol as a sole added carbon source. The Cr(Ⅵ) reduction ratios were 57.80%, 73.47%, 84.47%, 79.67%, 57.93% and 49.60% at initial phenol concentration of 25, 50, 100, 150, 200 and 250 mg/L, respectively. An increase in initial phenol concentration enhanced Cr(Ⅵ) reduction until an optimum value of 100 mg/L was reached. At the same time, the averaged phenol degradation rate increased from 0.39 to 1.52 mg/(L?h) when the initial phenol concentration increased from 25 to 150 mg/L. But further increase in initial phenol concentration from 150 to 200 mg/L led to the decrease in average phenol degradation rate from 1.52 to 1.48 mg/(L?h) (Fig.2(b)). Cr(Ⅵ) reduction was correlated to high organic acid formation from phenol degradation[16]. Organic acid formation accumulation might inhibit biological activity. At the same time, phenol and its ramification also inhibited the activity of the bacterial because of the protein denaturation. Phenol degradation in the culture produced a series of organic acid metabolite. High concentration of phenol inhibited biological activity, and then led to the accumulation of organic acid metabolite produced from phenol degradation, therefore, resulting in decrease in both Cr(Ⅵ) reduction and phenol degradation rates at last. Phenol concentration above the optimum level became an inhibit factor on Cr(Ⅵ) reduction and phenol degradation.
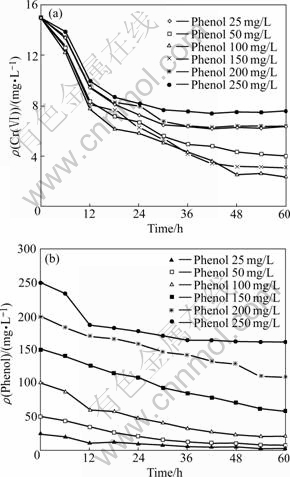
Fig.2 Effect of initial phenol concentration on Cr(Ⅵ) reduction (a) and phenol degradation (b) (Initial Cr(Ⅵ) concentration 15 mg/L and initial phenol concentration 25-250 mg/L)
3.4 Effect of cell composition
Both Cr(Ⅵ) reduction and phenol degradation were affected by the composition of cells. In this work, the effect of cell composition on Cr(Ⅵ) reduction and phenol degradation was investigated by varying the ratio of phenol-degraders (P. putida Migula) to Cr(Ⅵ)- reducers (Bacillus sp.). The data in Fig.3 and Fig.4 were tested under a constant initial Bacillus sp. density (cell wet mass 0.18 g) with varying P. putida Migula density and a constant initial P. putida Migula density (cell wet mass 0.20 g) with varying Bacillus sp. density, respectively. As shown in Figs.3(a) and (b), almost no Cr(Ⅵ) reduction or phenol degradation occurred in the culture without P. putida Migula. Phenol cannot be degraded if the culture lacks P. putida Migula. This means that the culture had no electron donors and energy source formed from phenol degradation to provide for Cr(Ⅵ) reduction, so both the phenol degradation and Cr(Ⅵ) reduction were insignificant. Increase in the ratio by Cr(Ⅵ) reduction decreased, and this then enhanced the phenol degradation due to the reduction of the toxic effects on phenol degradation. The correlation between substrate degradation and cell composition in the culture illustrated that the organic acid metabolites formed from phenol degradation became another limiting factor for Cr(VI) reduction.

Fig.3 Effect of varying cell composition of phenol-degraders in culture on Cr(Ⅵ) reduction (a), phenol degradation (b) and removal ratio (c) (P. putida Migula-to-Bacillus sp. ratio 0?1, 0.5?1, 1?1, 5?1 and 10?1)
of P. putida Migula to Bacillus sp. from 0?1 to 10?1 enhanced both the Cr(Ⅵ) reduction and phenol degradation (Fig.3(c)). Higher ratio of P. putida Migula to Bacillus sp. resulted in higher phenol degradation, more electron donor and energy source, indicating that the Cr(Ⅵ) reduction rate became larger. At the same time, the accumulation of organic acid metabolites consumed
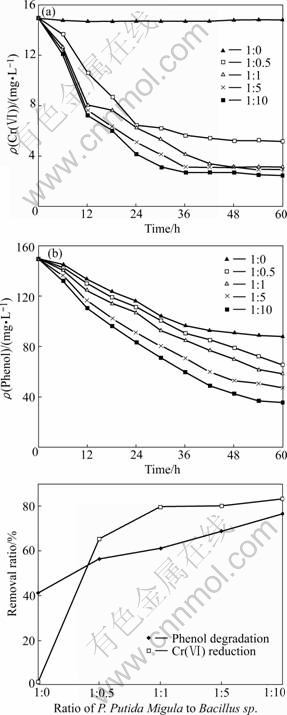
Fig.4 Effect of varying cell composition of Cr(Ⅵ)-reducers in culture on Cr(Ⅵ) reduction (a), phenol degradation (b) and removal ratio (c) (P. putida Migula-to-Bacillus sp. ratio 1?0, 1?0.5, 1?1, 1?5 and 1?10)
Fig.4 shows the effects of cell composition on Cr(Ⅵ) reduction and phenol degradation by varying ratio of P. putida Migula to Bacillus sp. from 1?0 to 1?10. Absolutely, no Cr(Ⅵ) reduction occurred in the culture without the presence of Bacillus sp. (Fig.4(a)). Increase in the proportion of initial Bacillus sp. concentration from 0 to 10?1 increased the reduction percentage of Cr(Ⅵ) from 1.26% to 83.67% (Figs.4(a) and (c)). However, the degradation percentage of phenol remained a rather stable increase trend under the same condition. This indicated that the phenol degradation was not significantly affected by Bacillus sp. Organic acid metabolite accumulation might inhibit phenol degradation. In this experiment, the more the quantity of Bacillus sp. that the culture contained, the faster the organic acid metabolites consumed, and then the more the phenol was degraded (Figs.4(b) and (c)).
3.5 Bioenergetics and mass balance
Theoretically, 5.14 mg Cr(Ⅵ) would be reduced per microgram of phenol degraded based on complete mineralization of phenol to water and bicarbonate:
C6H6O+9.3CrO42-+40.7H+=9.3Cr3++6HCO3-+20.3H2O
But, the highest efficiency of Cr(Ⅵ) reduction per unit mass of phenol degradation (0.37 mg Cr(Ⅵ)/mg phenol) was far less than that theoretically required (5.14 mg Cr(Ⅵ)/mg phenol). P. putida Migula oxidized phenol using molecular oxygen as an electron acceptor. Metabolites formed from the phenol degradation served as electron donor and energy source for Cr(Ⅵ) reduction since Bacillus sp. did not utilize phenol for growth directly. Therefore, the amount of phenol consumed was much more than that stoichiometrically required for Cr(VI) reduction because the energy produced from phenol degradation was not used by Cr(Ⅵ) reduction fully.
4 Conclusions
1) Simultaneous Cr(Ⅵ) reduction and phenol degradation using a coculture of P. putida Migula and Bacillus sp. was investigated.
2) Phenol was used as the sole carbon source, and the organic acid intermediates formed from the phenol degradation were used by Bacillus sp. for Cr(Ⅵ) reduction.
3) Cr(Ⅵ) had inhibiting effects on both phenol degradation and Cr(Ⅵ) reduction, whereas phenol enhanced both Cr(Ⅵ) reduction and phenol degradation until the optimum level was reached.
4) The composition of cell influenced the Cr(Ⅵ) reduction and phenol degradation. Phenol degradation and Cr(Ⅵ) reduction increased with increasing the ratio of P. putida Migula to Bacillus sp., and increase in the proportion of Bacillus sp. concentration in the culture also led to the increase of Cr(Ⅵ) reduction and phenol degradation.
5) The observed amount of phenol being degraded was more than that stoichiometrically required for Cr(Ⅵ) reduction.
References
[1] KOCBERBER N, D?NMEZ G. Chromium(Ⅵ) bioaccumulation capacities of adapted mixed culture isolated from industrial saline wastewaters [J]. Bioresource Tecnol, 2006, 8(17): 1-6.
[2] THACKER U, PARIKH R, SHOUCHE Y, MADAMWAR D. Hexavalent chromium reduction by providencia sp. [J]. Process Biochemistry, 2006, 41: 1332-1337.
[3] LIU Y G, XU W H, ZENG G M, LI X, GAO H. Cr(Ⅵ) reduction by Bacillus sp. isolated from chromium landfill [J]. Process Biochemistry, 2006, 41: 1981-1986.
[4] YASSI A, NIEBOER E. Chromium in natural and human environments [M]. New York: Wiley-Inerscience, 1998: 443-395.
[5] MERTZ W. Chromium as a dietary essential for man [M]. Baltimore: University Press, 1974: 185-198.
[6] GANGULI A, TRIPATHI A K. Bioremediation of toxic chromium from electroplating effluent by chromate-reducing Pseudomonas aeruginosa A2chr in two bioreactors [J]. Appl Microbiol Biotechnol, 2002, 58: 416-420.
[7] KONOVALOVA V V, DMYTRENKO G M, NIGMATULLIN R R, BRYK M T, GVOZDYAK P I. Chromium(Ⅵ) reduction in a membrane bioreactor with immobilized Psendomonas cells [J]. Enzyme Microb Technol, 2003, 33: 899-907.
[8] QUINTELAS C, FERNANDES B, CASTRO J, FIGUEIREDO H, TAVAVES T. Biosorption of Cr(Ⅵ) by three different bacterial species supported on granular activated carbon—A comparative study [J]. Journal of Hazardour Materials, 2008, 153: 799-809.
[9] ACKERLEY D F, GONZALEZ C F, KEYHAN M, BLAKE R, MATIN A. Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction [J]. Environ Microbiol, 2004, 6(8): 851-860.
[10] SARANGI A, KRISHNAN C. Comparison of in vitro Cr(Ⅵ) reduction by CFEs of chromate resistant bacterial isolated from chromate contaminated soil [J]. Bioresource Technol, 2008, 99: 4130-4137.
[11] GUHA H, JAYACHANDRAN K, MAURRASSE F. Kinetics of chromium(Ⅵ) reduction by a type strain Shewanella alga under different growth conditions [J]. Environ Pollut, 2001, 115: 209-218.
[12] VIAMAJALA S, PEYTON B M, PETERSEN J N. Modeling chromate reduction in Shewanella oneidensis MR-1: Development of a novel dual-enzyme kinetic model [J]. Biotechnol Bioeng, 2003, 83(7): 190-797.
[13] KONOVALOVA V V, DMYTRENKO G M, NIGMATULLIN R R, BRYK M T, GVOZDYAK P I. Chromium(Ⅵ) reduction in a membrane bioreactor with immobilized Pseudomonas cells [J]. Enzyme Microb Technol, 2003, 33: 899-907.
[14] LIU Y G, XU W H, ZENG G M, TANG C F, LI C F. Experimental study on Cr(Ⅵ) reduction by Pseudomunas aeruginosa [J]. J Environ Sci, 2004, 16 (5): 797-801.
[15] AKSU Z, G?NEN F. Binary biosorption of phenol and chromium(Ⅵ) onto immobilized activated sludge in a packed bed: Prediction of kinetic parameters and breakthrough curves [J]. Separation and Purification Technology, 2006, 49: 205-216.
[16] EVANS M, NKHALAMBAYAUSI C, WANG Y T. Simultaneous chromium(Ⅵ) reduction and phenol degradation in a fixed-film coculture bioreactor: Reactor performance [J]. Wat Res, 2001, 38(8): 1921-1932.
[17] KUO C W, GENTHNER B R S. Effect of added heavy metal ions on biotransformation and biodegradation of 2-chlorophenol and 3-chlorobenzoate in anaerobic bacterial consortia [J]. Appl and Environ Microbiology, 1996, 62(7): 2317-2323.
[18] SHEN H, PRITCHARD P H, SEWELL G W. Microbial reduction of Cr(Ⅵ) during anaerobic degradation of benzoate [J]. Environ Sci Technol, 1996, 30: 1667-1674.
[19] WANG Y T, CHIRWA E M. Simultaneous removal of Cr(Ⅵ) and phenol in chemostat culture of E. coli ATCC 33456 and P. putida DMP-1 [J]. Wat Sci Tech, 1998, 38(8/9): 113-119.
[20] MAZIERSKI J. Effect of chromium (CrVI) on the growth rate of denitrifying bacteria [J]. Wat Res, 1994, 28: 1981-1985.
[21] SHEN H, WANG Y T. Biological reduction of chromium by E.coli [J]. J Environ Eng, 1994, 120: 560-572.
Foundation item: Project(04JJ3013) supported by the Natural Science Foundation of Hunan Province, China; Project(20050532009) supported by the Doctoral Foundation of Ministry of Education of China; Project supported by the National Foundation of Student Innovation Training, China
Corresponding author: LIU Yun-guo; Tel: +86-731-8649208; E-mail: liuyunguo@hnu.cn
(Edited by YANG Bing)


