
Optimization of processing parameters for preparation of Al-3Ti-1B grain refiner
LI Bao(李 豹)1, WANG Hong-wei(王宏伟)1, ZHAO Rui-feng(赵瑞峰)2, WEI Zun-jie(魏尊杰)1
1. School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, China;
2. Beijng Xinghang Mechanical Electrical Equipment Factory, Beijing 100074, China
Received 10 June 2009; accepted 15 August 2009
Abstract:
Al-3Ti-1B master alloys were prepared at different processing parameters by the reaction of halide salts, and the grain refining response of Al-7Si alloy was investigated with Al-3Ti-B master alloy. The microstructure of master alloy and its grain refining effect on Al-7Si alloy were investigated by means of OM, XRD and SEM. Experimental results show that, the size of Al3Ti particles presented in Al-3Ti-1B master alloys increases with the increase of reaction temperature and decreases with the increase of cooling rate. The grain refining efficiency of Al-3Ti-1B master alloy on Al-7Si alloy is mainly attributed to heterogeneous nucleation of Al3Ti particles, and the morphology of α(Al) changes from coarse dendritic to fine equiaxed. As a result, Al-3Ti-1B master alloy is prepared by permanent mold, and holding at 800 ℃ for 30 min, which has better grain refining performance on Al-7Si alloy.
Key words:
Al-3Ti-1B master alloy; processing parameters; grain refinement; Al-7Si alloy;
1 Introduction
The hypoeutectic Al-Si alloys are extensively used in the automotive and aerospace industries because of the excellent properties including castability, weldability, corrosion resistance and integrated mechanical properties[1]. The quality of hypoeutectic Al-Si alloys can be improved by grain refinement that reduces the size of primary α(Al) grains in the casting, which otherwise solidifies with coarse columnar grain structure[2-3]. A fine equiaxed grain structure leads to several benefits, such as high yield strength, high toughness, improved machinability and excellent deep drawability of the products[4-5].
Grain refinement can be achieved by different means involving fast cooling, heterogeneous nucleation, solute addition and melt agitation[6-7]. Al-Ti-B type master alloys are added to the melt in order to introduce potent particles, Al3Ti and TiB2, which are effective nucleant substrates for the primary α(Al) phase. Although many studies of grain refinement have been performed, these have mostly considered the action, mechanism and development of commercial grain refiners[8-9]. Production of Al-Ti-B master alloys involves reaction of halide salts with molten aluminum[10], equal-channel angular pressing (ECAP) [11] and powder metallurgy (PM)[12-13]. The addition of halide salts (K2TiF6-KBF4) to molten Al has become popular. While commercially very popular, the “halide salt” route suffers several drawbacks in practice[5, 14], and the problems are still encountered in the cast house. Moreover, with a high silicon content, a silicide can form on the TiB2 particles in place of the Al3Ti layer preventing or decreasing the probability of nucleation of aluminum grains on the particles[15]. And the Ti/B mass ratios of the grain refiner affect their performance in Al-7Si alloys, when Ti/B mass ratio is more than 3 also it could refine the grains but at high Ti levels[16]. Otherwise, the results obtained in regard to grain refinement with master alloys thus produced, differ appreciably, since their microstructure and performance as a grain refiner are highly sensitive to the processing parameters used in production[17]. The reaction temperature plays a critical role in the manufacturing of Al-Ti-C and Al-Ti-B master alloys. The size, distribution and morphologies of TiC are influenced mainly by the synthesis temperature and holding time[18]. Higher reaction temperature promotes the growth of the nucleating particles, thereby resulting in change in morphology[19].
In this work, the influence of various processing parameters such as reaction temperature and time, cooling conditions on the microstructure of the Al-3Ti-1B master alloys was studied. Meantime, Al-3Ti-1B grain refining behavior of hypoeutectic Al-7Si alloys was investigated.
2 Experimental
The Al-3Ti-1B master alloys were prepared in a resistance furnace by the combined addition of preheated halide salts (K2TiF6 and KBF4) to the molten Al at different processing parameters. The processing parameters for the preparation of master alloys are shown in Table 1. Once the molten Al reached the required temperature, halide salts weighed in required propositions were added to the melt, and the melt was poured into different mold when the holding time reached.
For grain refinement studies, Al-7Si alloy was prepared from commercial purity Al (99.7%) and Al-12Si master alloy. Al-7Si alloys were melted in a 15 kW resistance furnace and the melt was held at 720 ℃. After degassing with high purity argon gas for 30 min through graphite rod immersed into the melt, the Al-3Ti-1B master alloy chips were added to the melt for grain refinement. The addition level of the master alloy kept constant at 0.2%Ti (mass fraction). Al-3Ti-1B master alloys and grain refined samples were characterized by optical microscopy and SEM micro- analyser using Poulton’s reagent. Tensile test of the specimens was carried out on Instron1186 Testing Machine.
3 Results and discussion
3.1 Microstructure of Al-3Ti-1B master alloys
The size, morphology and distribution of particles in Al-3Ti-1B master alloys prepared at different processing parameters are shown in Fig.1. Figs.1(a)-(i) show the microstructures of the master alloys prepared according to ortho-experiments in Table 1. X-ray
Table 1 Factors-levels of ortho-experiments L9(34)
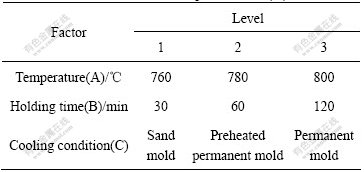
Fig.1 SEM morphologies of Al-3Ti-1B master alloys obtained under different experimental conditions: (a) A1, B1, C1; (b) A1, B2, C2; (c) A1, B3, C3; (d) A2, B1, C3; (e) A2, B2, C1; (f) A2, B3, C2; (g) A3, B1, C2; (h) A3, B2, C3; (i) A3, B3, C1
diffraction studies reveal the presence of Al3Ti, Ti2B and α(Al) phases in Al-3Ti-1B master alloys. In addition, Fig.1 also reveal the presence of Al3Ti particles (light grey phase) in Al-3Ti-1B master alloys, whereas Ti2B particles are invisible for the little quantity.
The SEM studies show predominantly blocky and plate-like morphology of Al3Ti particles in all the master alloys. It is clear from Fig.1 that the sizes of Al3Ti particles presented in Al-3Ti-1B master alloys increase with the increase of reaction temperature. Such increase in particle sizes of the master alloys suggests that, higher reaction temperature promotes the growth of the particles. Meantime, the Ti recovery increases with the increase of reaction temperature. At reaction temperature of 760 ℃, the Ti recovery in Al-3Ti-1B master alloy is about 67%, while at 780 ℃ and 800 ℃ it increases to 86.5% and 94.5%, respectively. It is apparent that the reaction temperature of 800 ℃ shows maximum recovery of Ti compared to the reaction temperatures of 760 ℃ and 780 ℃. As a result, the number of Al3Ti particles which acts as heterogeneous nucleating sites during solidification is large at the reaction temperatures of 800 ℃.
Refined blocky morphology of the Al3Ti particles are observed in permanent mold with rapid cooling rate. However, at all holding times, the size and morphology of Al3Ti particles remained the same, therefore, the influence of holding time on the size and morphology of Al3Ti particles can be ignored. Moreover, a fairly uniform distribution of Al3Ti particles is seen in the master alloys prepared at 800 ℃ and clustering of the particles is observed in the others.
3.2 Grain refining efficiency of Al-3Ti-1B master alloys on Al-7Si alloy
Fig.2 represents the photomacrographs of Al-7Si alloy after the addition of Al-3Ti-1B master alloys prepared at different processing parameters. It is clear from Fig.2 that with the addition of 0.2% Ti, Al-7Si alloy shows response towards grain refinement in all conditions. Such structural changes could be due to the
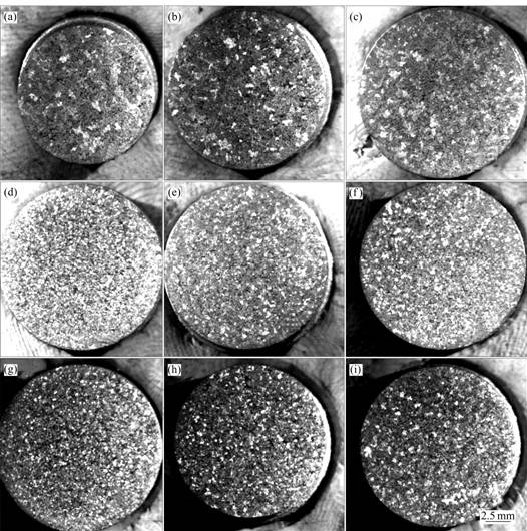
Fig.2 Photomacrographs of Al-7Si alloy after addition of Al-3Ti-1B master alloys obtained under different experimental conditions: (a) A1, B1, C1; (b) A1, B2, C2; (c) A1, B3, C3; (d) A2, B1, C3; (e) A2, B2, C1; (f) A2, B3, C2; (g) A3, B1, C2; (h) A3, B2, C3; (i) A3, B3, C1
presence of Al3Ti particles presented in Al-3Ti-1B master alloy, which acts as heterogeneous nucleating sites during solidification.
From the photomacrographs in Fig.2, we could clearly see that Al-3Ti-1B master alloys prepared at higher reaction temperature show better grain refining efficiency compared to the one prepared at low reaction temperature, and Al-7Si alloy shows response towards grain refinement with structural transition from columnar to equiaxed structure in Fig.3. This difference in grain refining efficiency could be attributed to the size, morphology and distribution of Al3Ti particles presented in the master alloys as discussed earlier. However, the influences of cooling rate and holding time on the grain refining behavior of the master alloy can be neglected compared to the reaction temperature.
With the addition of 0.2%Ti (Al-3Ti-1B) to Al-7Si alloy, the structure changes from elongated coarse columnar to fine equiaxed α(Al) dendritic structure, such structural conversion is due to the Al3Ti particles presented in Al-3Ti-1B master alloy, which acts as heterogeneous nucleating sites. This change in microstructure leads to improvement in mechanical properties. Table 2 shows the tensile testing results of Al-7Si alloys refined by different Al-3Ti-1B refiners. Tables 3 and 4 show the corresponding range analysis of the results, respectively.
Table 2 Results of tensile testing
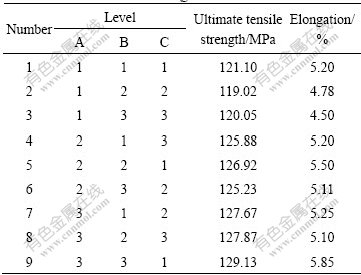
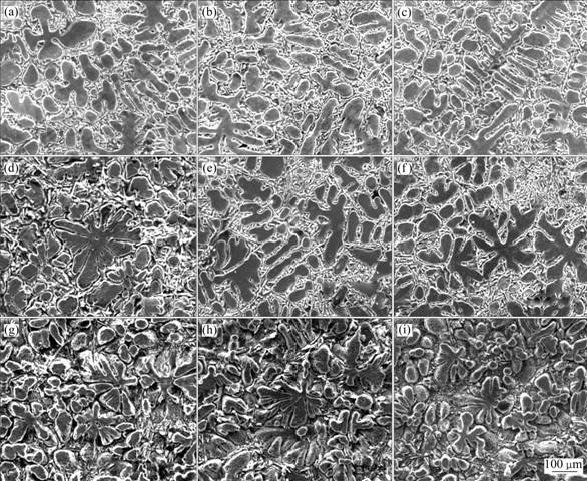
Fig.3 SEM microphotographs of Al-7Si alloy after addition of master alloys obtained under different experimental conditions: (a) A1, B1, C1; (b) A1, B2, C2; (c) A1, B3, C3; (d) A2, B1, C3; (e) A2, B2, C1; (f) A2, B3, C2; (g) A3, B1, C2; (h) A3, B2, C3; (i) A3, B3, C1
Table 3 Range analysis of ultimate tensile strength (MPa)
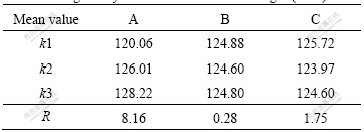
Table 4 Range analysis of elongation (%)
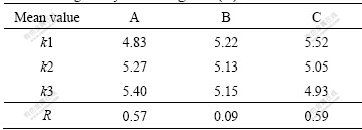
It can be seen from Tables 3 and 4 that, the influence sequence of the processing parameters is as follows: reaction temperature, cooling rate and holding time. The ultimate tensile strength increases with the increase of reaction temperature, and the grain refining efficiency is favorable to Al-Si alloys by increasing cooling rate. As a result, Al-3Ti-1B master alloy that is prepared by permanent mold, and holding at 800 ℃ for 30 min, has better grain refining efficiency on Al-7Si alloy.
4 Conclusions
1) The processing parameters play critical role in the manufacturing of Al-3Ti-1B master alloys and influence the size, morphology and distribution of Al3Ti particles presented in Al-3Ti-1B master alloys. The size of Al3Ti particles increases with the increase of reaction temperature and decreases with increasing cooling rate. However, the influence of holding time on the size and morphology of Al3Ti particles can be ignored.
2) The grain refining efficiency of Al-3Ti-1B master alloy on Al-7Si alloy is mainly attributed to heterogeneous nucleation of Al3Ti particles. The size, morphology and distribution of Al3Ti particles strongly influence the grain refining behavior of the master alloy. As a result, Al-3Ti-1B master alloy that is prepared by permanent mold, and holding at 800 ℃ for 30 min, has better grain refining response in Al-7Si alloy.
References
[1] MCCARTNEY D G, HUNT J D. Measurements of cell and primary dendrite arm spacings in directionally solidified aluminium alloys[J]. Acta Metallurgica, 1981, 29 (11): 1851-1863.
[2] JUNG H, MANGELINCK-NO?L N, BERGMAN C, BILLIA B. Determination of the average nucleation undercooling of primary Al-phase on re?ning particles from Al-5.0 wt% Ti-1.0wt% B in Al-based alloys using DSC[J]. Journal of Alloys and Compounds, 2009, 477: 622-627.
[3] LEE Y C, DAHLE A K, STJOHN D H, HUTT J E C. The effect of grain refinement and silicon content on grain formation in hypoeutectic Al-Si alloys[J]. Materials Science and Engineering A, 1999, 259: 43-52.
[4] VINOD-KUMARA G S, MURTYB B S, CHAKRABORTYA M. Grain re?nement response of LM25 alloy towards A-Ti-C and Al-Ti-B grain re?ners[J]. Journal of Alloys and Compounds, 2009, 472: 112-120.
[5] KORI S A, MURTY B S. Development of an efficient grain refiner for Al-7Si alloy and its modification with strontium[J]. Materials Science and Engineering A, 2000, 283: 94-104.
[6] ZHANG M X, KELLY P M, EASTON M A, TAYLOR J A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model[J]. Acta Materialia, 2005, 53(5): 1427-1438.
[7] MAYES C D, MCCARTNEY D G, TATLOCK G J. Observations on the microstructure and performance of an Al-Ti-C grain-refining master alloy[J]. Materials Science and Engineering A, 1994, 188(1/2): 283-290.
[8] MOHANTY P S, GRUZLESKI J E. Mechanism of grain refinement in aluminium[J]. Acta Metallurgica Materialia, 1995, 43(5): 2001-2012.
[9] KORI, S A, MURTY B S, CHAKRABORTY M. Development of an efficient grain refiner for Al-7Si alloy[J]. Materials Science and Engineering A, 2000, 280(1): 58-61.
[10] BIROL Y. An improved practice to manufacture Al-Ti-B master alloys by reacting halide salts with molten aluminum[J]. Journal of Alloys and Compounds, 2006, 420: 71-76.
[11] ZHANG Z, HOSODA S, KIM I, WATANABE Y. Grain re?ning performance for Al and Al-Si alloy casts by addition of equal-channel angular pressed Al-5 mass%Ti alloy[J]. Materials Science and Engineering A, 2006, 425: 55-63.
[12] CARDOSO K R, RODRIGUES C A D, BOTTA W J. Processing of aluminum alloys containing titanium addition by mechanical alloying[J]. Materials Science and Engineering A, 2004, 375: 1201-1205.
[13] BIROL Y. Al-Ti-B grain re?ners via powder metallurgy processing of Al/K2TiF6/KBF4 powder blends[J]. Journal of Alloys and Compounds, 2009, 480: 311-314.
[14] BIROL Y. Production of Al-Ti-B master alloys from Ti sponge and KBF4[J]. Journal of Alloys and Compounds, 2007, 440: 108-112.
[15] GROBNER J, MIRKOVIC D, SCHMID-FETZER R. Thermo- dynamic aspects of grain refinement of Al-Si alloys using Ti and B[J]. Materials Science and Engineering A, 2005, 395: 10-21.
[16] SRITHARAN T, LI H. Influence of titanium to boron ratio on the ability to grain refine aluminium-silicon alloys[J]. Journal of Materials Processing Technology, 1997, 63(1/3): 585-589.
[17] MURTY B S, KORI S A, VENKATESWARLU K, BHAT R R, CHAKRABORTY M. Manufacture of Al-Ti-B master alloys by the reaction of complex halide salts with molten aluminium[J]. Journal of Materials Processing Technology, 1999, 89/90: 152-158.
[18] DING Hai-min, LIU Xiang-fa, YU Li-na, ZHAO Guo-qun. The influence of forming processes on the distribution and morphologies of TiC in Al-Ti-C master alloys[J]. Scripta Materialia, 2007, 57: 575-578.
[19] AURADI V, KORI S A. Influence of reaction temperature for the manufacturing of Al-3Ti and Al-3B master alloys[J]. Journal of Alloys and Compounds, 2008, 453(1/2): 147-156.
(Edited by YANG You-ping)
Corresponding author: LI Bao; Tel: +86-451-86403150; E-mail: frodo-lee@163.com


